Adsorption and removal mechanism of atomic oxygen on different facets of θ-Fe3C
-
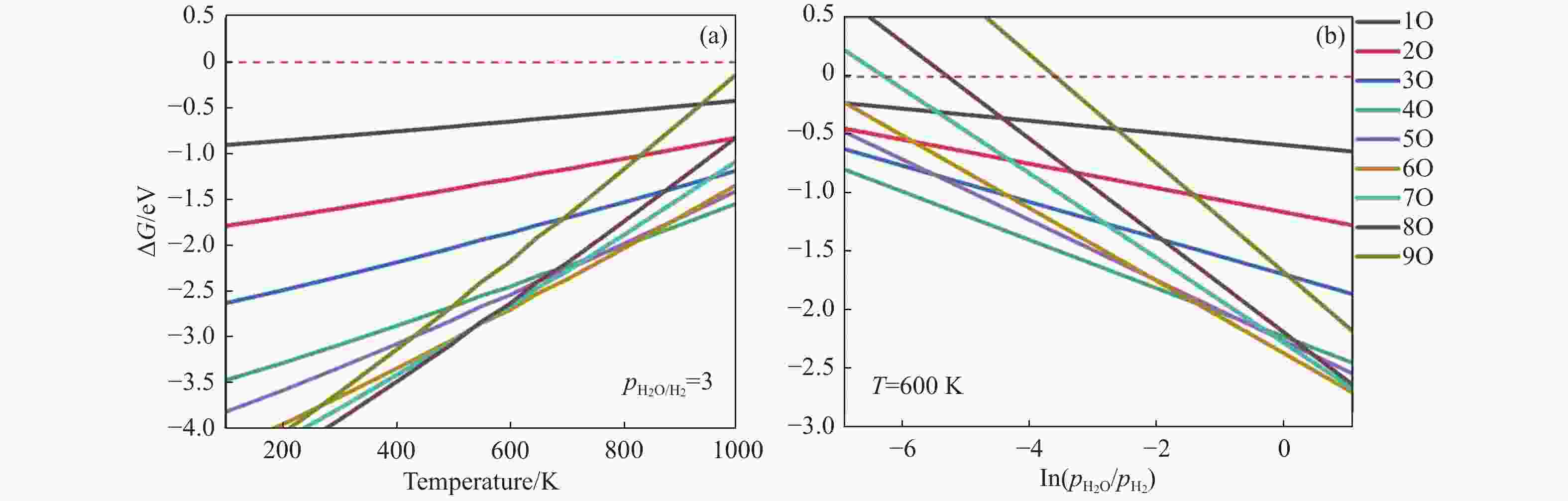
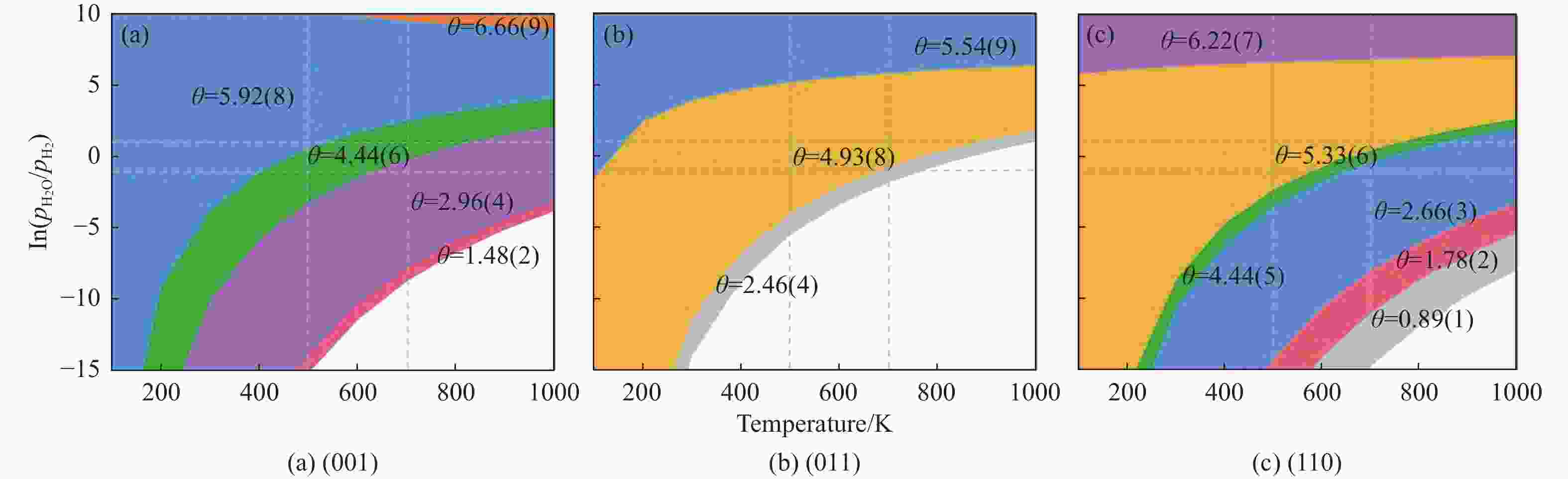
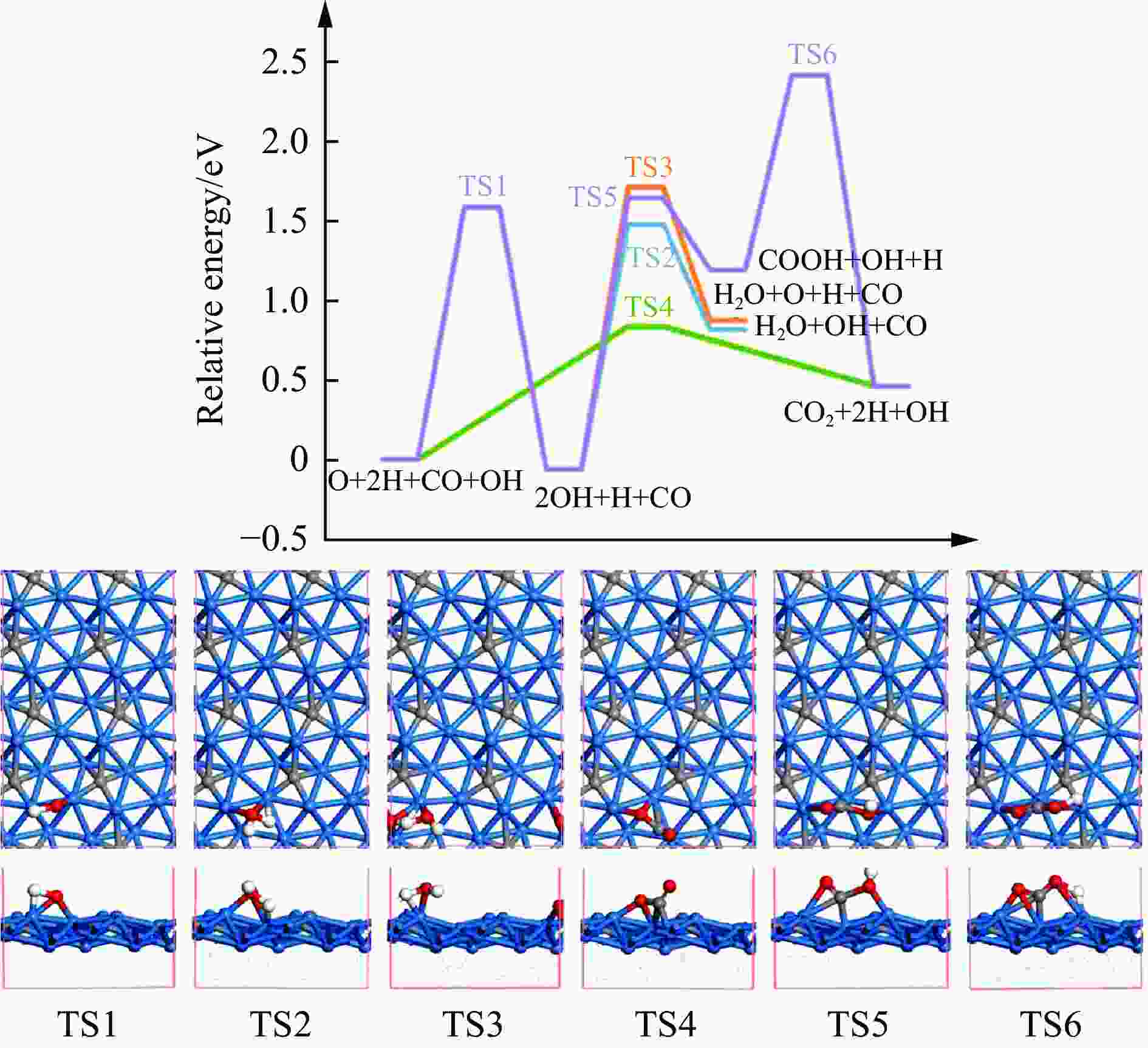
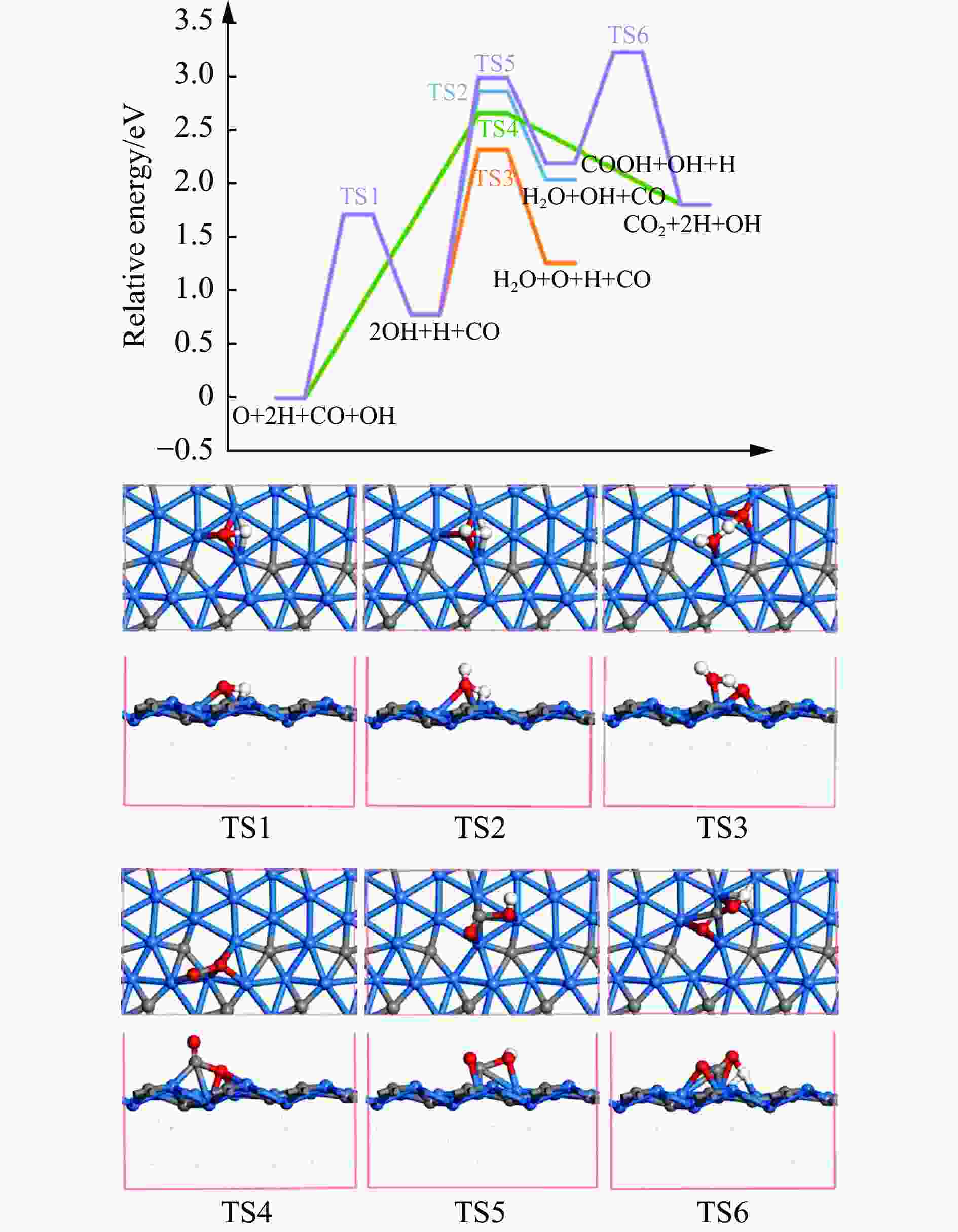
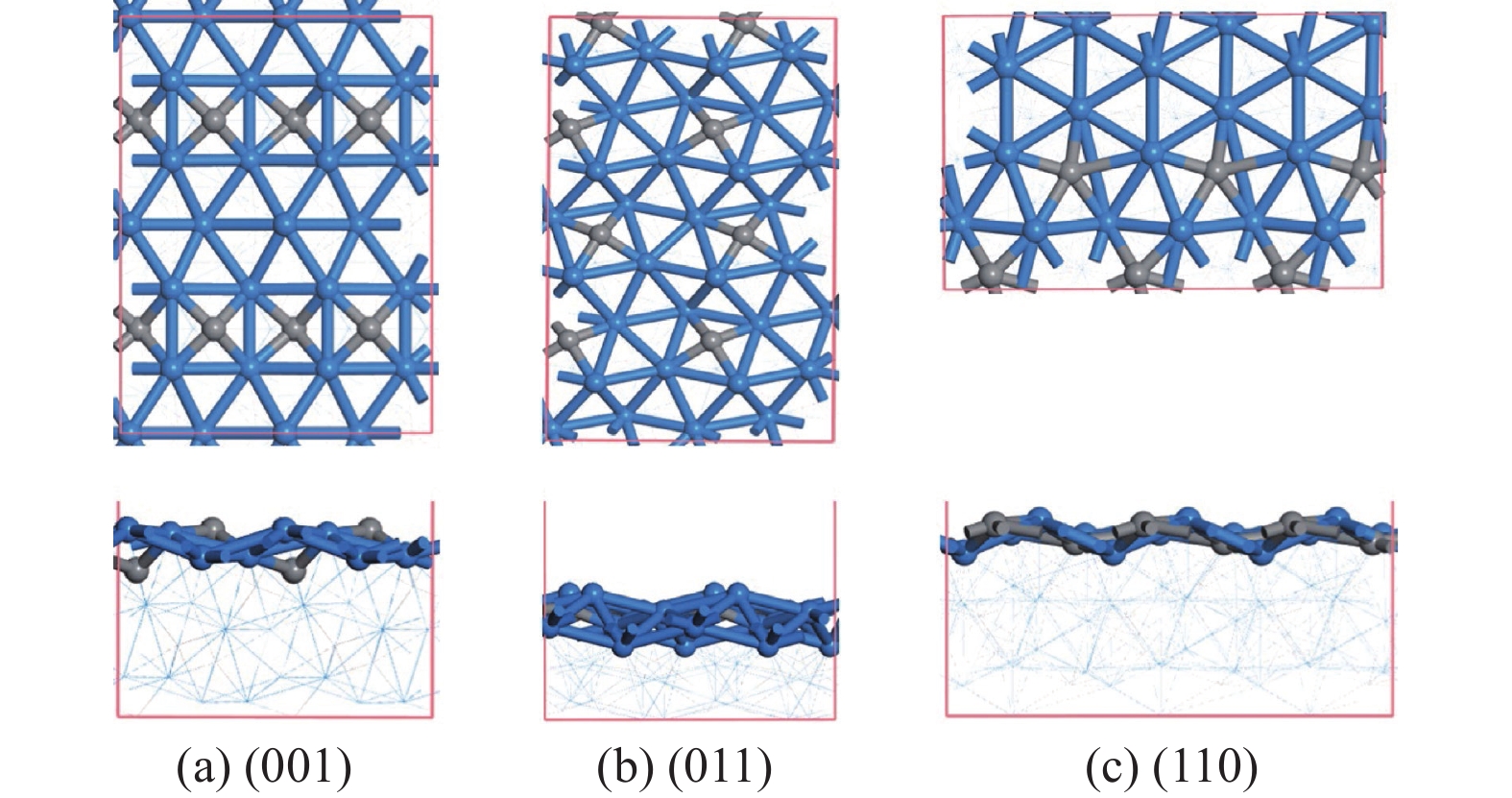
摘要: 碳化铁θ-Fe3C作为费托反应活性相之一,其氧化造成了催化剂的严重失活。探究氧原子在碳化铁晶面的吸附及移除机理有助于理解氧化过程,为提高催化剂的稳定性提供参考。本工作通过理论计算研究了低覆盖度下氧原子在θ-Fe3C不同晶面的吸附,其在(110)晶面吸附最强,(001)晶面次之,(011)晶面吸附最弱,即(110)容易氧化。原子热力学研究表明,增大H2O分压或降低温度有利于氧原子吸附,容易造成表面氧化。此外,在典型费托反应条件下(110)晶面氧原子的覆盖度最高,进一步证明该晶面易氧化,与低覆盖度吸附结果一致。对移除路径进行计算得出,(011)晶面吸附氧原子直接与CO反应以CO2方式移除能垒较低(0.84 eV);(001)与(110)晶面吸附氧原子主要通过OH歧化以H2O方式移除,但后者形成O-H键需要克服的能垒更高(1.72 vs 1.47 eV)。Abstract: The Fisher-Tropsch Synthesis (FTS) is one of the most intensively studied reactions in heterogeneous catalysis, which could convert the syngas (CO and H2) from coal, natural gas, shale gas, and biomass into gasoline, diesel, and a series of important chemical products. The process provides an effective strategy for reducing environmental pollution caused by direct coal combustion, at the meanwhile, alleviating dependence on imported petroleum. During the reaction, iron-based catalysts have attracted the attention of numerous researchers due to their unique advantages, for example, low cost, flexibility in product distribution, suitability for low H2/CO ratio syngas and large operating space. And the iron-based catalysts in FTS have achieved applications successfully in industry. Iron carbide is recognized as active phases in FTS catalyzed by iron-based catalysts, among which θ-Fe3C is one of the dominant phases. Additionally, θ-Fe3C is widely applied in fields such as biomedicine and electrochemistry. However, θ-Fe3C could be oxidized under realistic conditions, affecting the performance as magnetic materials and catalysts seriously. Considering the case above, the investigation of oxidation of iron carbide θ-Fe3C is of great significance. And exploring the adsorption, as well as removal mechanism of atomic oxygen over the iron carbide facet is helpful for understanding the oxidation process, and providing a reference for improving the stability of catalysts. In this work, we have explored the adsorption of oxygen atoms from low to high coverage on three θ-Fe3C surfaces with density functional theory. Ab initio atomistic thermodynamics was utilized to investigate the effect of experimental conditions like temperature and partial pressure of H2O. According to the calculation, it was found that the adsorption at low coverage on (110) was the strongest, followed by (001), and the adsorption on (011) was the weakest, meaning that (110) can be oxidized easily. As the number of oxygen atoms adsorbed on the surface increases, the stepwise adsorption energy increases, which is a manifestation of the repulsive effect between adsorbed oxygen atoms. The average adsorption energy for each surface increases with the increase of adsorbed oxygens, and the magnitude of increase varies due to the different local structures of each facet. The average adsorption energy on (011) has a relatively small increase, indicating that the repulsion between oxygen atoms adsorbed on this crystal plane is weak; whereas opposite on (001) and (110) facets. Atomistic thermodynamic studies showed that increasing the partial pressure of H2O or decreasing the temperature will stabilize the O adsorption, leading to surface oxidation. In addition, the highest O coverage on (110) under typical FTS conditions further proved that the facet is easily oxidized, which is consistent with the adsorption results at low coverage. Finally, the removal path of adsorbed oxygen on different facets was calculated, and the results showed that adsorbed O on (011) prefer to react with CO with energy barrier of 0.84 eV. On (001) and (110), removal in the form of H2O via OH disproportionation is more favored, but the energy barrier to form O−H bond is higher for the latter facet (1.72 vs 1.47 eV).
-
Key words:
- θ-Fe3C /
- oxidation /
- adsorption of atomic O /
- removal mechanism
-
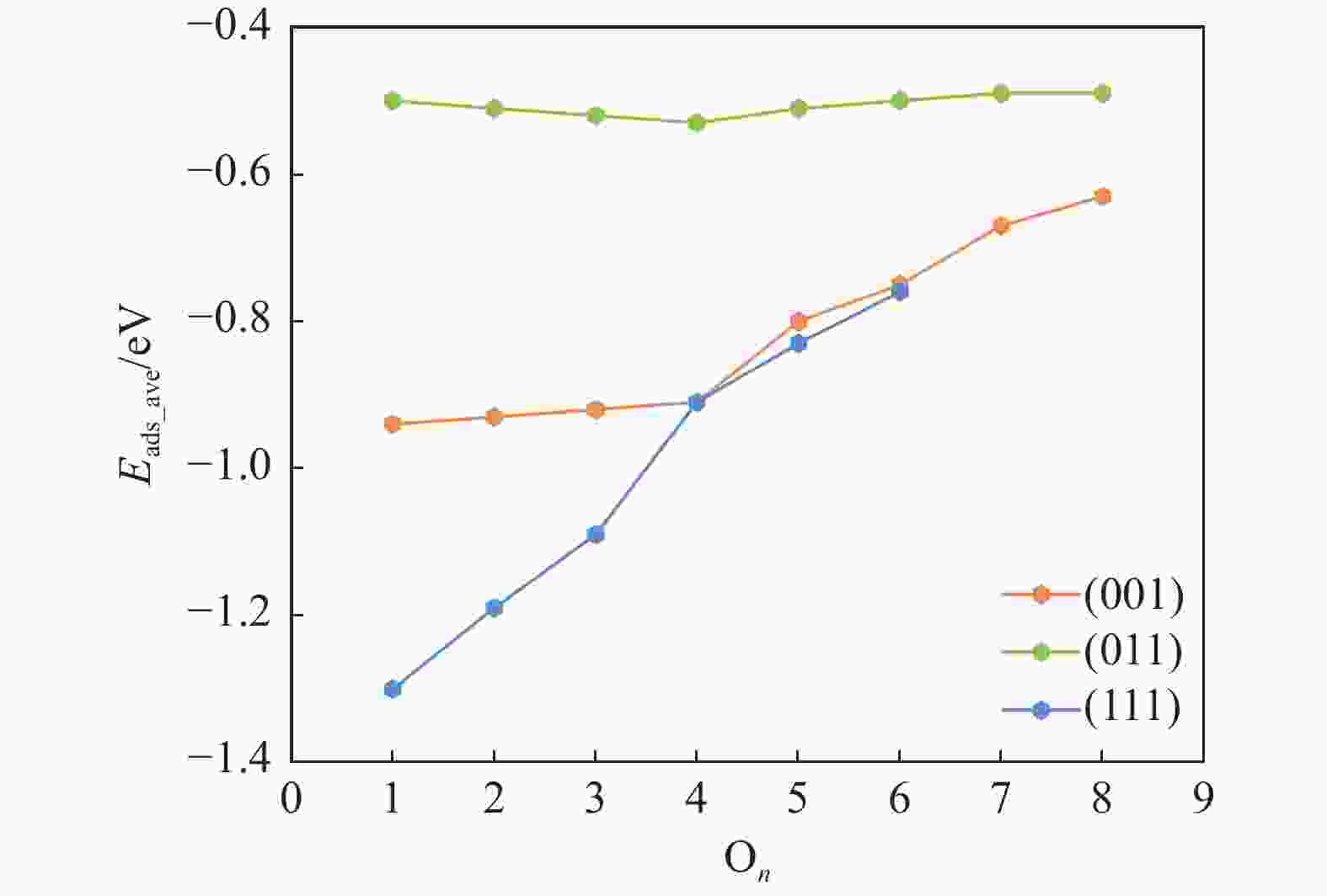
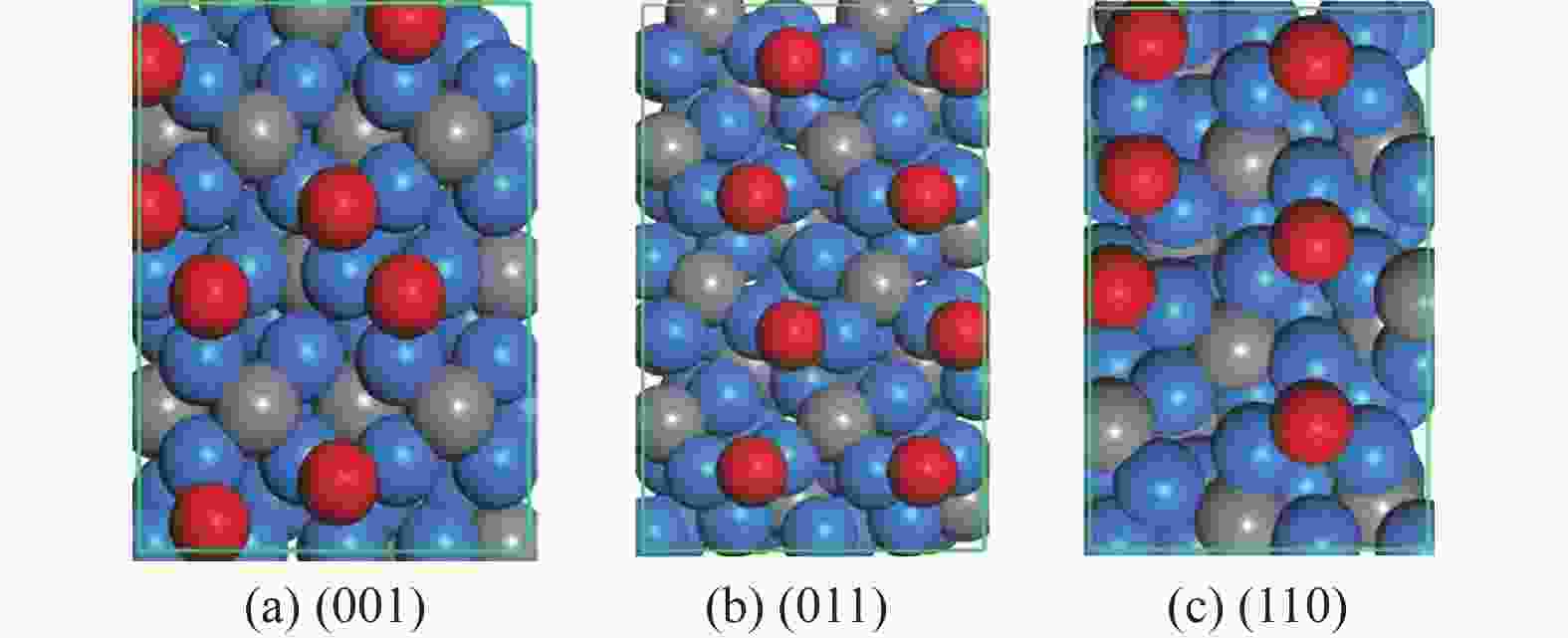
表 1 氧原子在各晶面吸附的相关信息,包括吸附个数(On)、吸附位点 (site)及逐步吸附能 (∆Eads, eV)
Table 1 Information of O adsorption on three surfaces, including the number of adsorbed O (On), adsorption site and stepwise adsorption energy (∆Eads)
Surface On Site ∆Eads (001) 1 3F −0.94 2 3F −0.93 3 3F −0.89 4 3F −0.88 5 3F −0.39 6 3F −0.46 7 3F −0.26 8 3F −0.26 9 CTa 0.16 (011) 1 B −0.50 2 B −0.53 3 B −0.52 4 B −0.56 5 B −0.44 6 B −0.46 7 B −0.46 8 B −0.48 9 3F 0.06 (110) 1 3F −1.30 2 3F −1.08 3 3F −0.90 4 3F −0.35 5 B −0.52 6 B −0.39 7 3F 0.00 CT site indicates that oxygen atoms are adsorbed on the carbon atom on the surface. 表 2 Fe3C各晶面氧原子移除相关基元反应的能垒和反应能
Table 2 The activation energy (Ea, eV) and reaction energy(Er, eV) of elementary reactions involved in the removal route
Reactions (001) (011) (110) O+H→OH Ea 1.47 1.59 1.72 Er 0.56 −0.06 0.78 OH+H→H2O Ea 1.75 1.54 2.09 Er 1.00 0.88 1.26 2OH→H2O+O Ea 0.89 1.77 1.54 Er 0.43 0.93 0.48 CO+O→CO2 Ea 2.18 0.84 2.67 Er 0.85 0.46 1.81 CO+OH→COOH Ea 1.70 1.70 2.22 Er 1.12 1.25 1.42 COOH→CO2+H Ea 0.63 1.23 1.04 Er −0.83 −0.73 −0.39 -
[1] YANG C, ZHAO H, HOU Y, et al. Fe5C2 nanoparticles: a facile bromide-induced synthesis and as an active phase for Fischer-Tropsch synthesis[J]. J Am Chem Soc,2012,134(38):15814−15821. doi: 10.1021/ja305048p [2] NIU L, LIU X, ZHOU X, et al. Genesis of an Fe5C2@Fe3O4 core/shell structure during CO carburization of metallic iron nanoparticles[J]. J Catal,2022,407:97−103. doi: 10.1016/j.jcat.2022.01.029 [3] CHEN Y, WEI J, DUYAR M S, et al. Carbon-based catalysts for Fischer-Tropsch synthesis[J]. Chem Soc Rev,2021,50(4):2337−2366. doi: 10.1039/D0CS00905A [4] PHAM T H, QI Y, YANG J, et al. Insights into Hägg iron-carbide-catalyzed Fischer-Tropsch synthesis: Suppression of CH4 formation and enhancement of C-C coupling on χ-Fe5C2(510)[J]. ACS Catal,2015,5(4):2203−2208. doi: 10.1021/cs501668g [5] XIONG H, JEWELL L L and COVILLE N J. Shaped carbons as supports for the catalytic conversion of syngas to clean fuels[J]. ACS Catal,2015,5(4):2640−2658. doi: 10.1021/acscatal.5b00090 [6] LIU Z-P and HU P. A New insight into Fischer-Tropsch synthesis[J]. J Am Chem Soc,2002,124:11568−11569. doi: 10.1021/ja012759w [7] JANBROERS S, LOUWEN J, ZANDBERGEN H, et al. Insights into the nature of iron-based Fischer-Tropsch catalysts from quasi in situ TEM-EELS and XRD[J]. J Catal,2009,268(2):235−242. doi: 10.1016/j.jcat.2009.09.021 [8] 温晓东, 杨勇, 相宏伟等. 费托合成铁基催化剂的设计基础: 从理论走向实践[J]. 中国科学: 化学,2017,47(11):1298−1311. doi: 10.1360/N032017-00111WEN Xiaodong, YANG Yong, XIANG Hongwei, et al. The design principle of iron-based catalysts for fischer-tropsch synthesis: from theory to practice[J]. Sci Sin Chim,2017,47(11):1298−1311. doi: 10.1360/N032017-00111 [9] BARRIOS A J, GU B, LUO Y, et al. Identification of efficient promoters and selectivity trends in high temperature Fischer-Tropsch synthesis over supported iron catalysts[J]. Appl Catal B: Environ,2020,273:119028. doi: 10.1016/j.apcatb.2020.119028 [10] TANG L, HE L, WANG Y, et al. Selective fabrication of χ-Fe5C2 by interfering surface reactions as a highly efficient and stable Fischer-Tropsch synthesis catalyst[J]. Appl Catal B: Environ,2021,284:119753−119762. doi: 10.1016/j.apcatb.2020.119753 [11] CHEN B, WANG D, DUAN X, et al. Charge-tuned CO activation over a χ-Fe5C2 Fischer-Tropsch catalyst[J]. ACS Catal,2018,8:2709−2714. doi: 10.1021/acscatal.7b04370 [12] LU F, CHEN X, LEI Z, et al. Revealing the activity of different iron carbides for Fischer-Tropsch synthesis[J]. Appl Catal B: Environ,2021,281:119521−119531. doi: 10.1016/j.apcatb.2020.119521 [13] OPEYEMI OTUN K, YAO Y, LIU X, et al. Synthesis, structure, and performance of carbide phases in Fischer-Tropsch synthesis: A critical review[J]. Fuel,2021,296:120689-. doi: 10.1016/j.fuel.2021.120689 [14] DE SMIT E, CINQUINI F, BEALE A M, et al. Stability and reactivity of ϵ-χ-ϴ iron carbide catalyst phases in Fischer-Tropsch synthesis: Controlling μc[J]. J Am Chem Soc,2010,132(42):14928−14941. doi: 10.1021/ja105853q [15] ZHANG M, REN J and YU Y. Insights into the hydrogen coverage effect and the mechanism of Fischer-Tropsch to olefins process on Fe5C2(510)[J]. ACS Catal,2019,10(1):689−701. [16] 何富贵, 张曈, 梁洁等. DFT计算在铁基催化剂费托合成反应研究中的应用[J]. 燃料化学学报, 2023, 51 (11) : 1540-1564.HE Fugui, ZHANG Tong, LIANG Jie, et al. Application of DFT calculation in the study of iron-based catalyst for Fischer-Tropsch synthesis[J]. J Fuel Chem Technol, 2023, 51 (11) : 1540-1564.) [17] TU W, SUN C, ZHANG Z, et al. Chemical and structural properties of Na decorated Fe5C2-ZnO catalysts during hydrogenation of CO2 to linear α-olefins[J]. Appl Catal B: Environ,2021,298:120567−120578. doi: 10.1016/j.apcatb.2021.120567 [18] WANG J, CAO M, XU F, et al. A cage compound precursor-derived Sb/Sb2O4/Fe3C nanocomposite anchored on reduced graphene oxide as an anode for potassium ion batteries[J]. New J Chem,2021,45(2):993−1000. doi: 10.1039/D0NJ05160H [19] ZHANG C, CAO C, ZHANG Y, et al. Unraveling the role of zinc on bimetallic Fe5C2-ZnO catalysts for highly selective carbon dioxide hydrogenation to high carbon α-olefins[J]. ACS Catal,2021,11(4):2121−2133. doi: 10.1021/acscatal.0c04627 [20] ZHANG Z, YIN H, YU G, et al. Selective hydrogenation of CO2 and CO into olefins over sodium- and zinc-promoted iron carbide catalysts[J]. J Catal,2021,395:350−361. doi: 10.1016/j.jcat.2021.01.036 [21] ZHAO X Q, LIU B X, LIANG Y, et al. Oxidation behavior and magnetic properties of metallic ultrafine particles[J]. J Magn Magn Mater,1996,164:401−410. doi: 10.1016/S0304-8853(96)00457-X [22] DE SMIT E, WECKHUYSEN B M. The renaissance of iron-based Fischer-Tropsch synthesis: on the multifaceted catalyst deactivation behaviour[J]. Chem Soc Rev,2008,37(12):2758−2781. doi: 10.1039/b805427d [23] CALVIN H. BARTHOLOMEW, MATTHEW W. STOKER, LINDA MANSKER, et al. Effects of pretreatment, reaction, and promoter on microphase structure and Fischer-Tropsch activity of precipitated iron catalysts[J]. Stud Surf Sci Catal,1999,126:265−272. [24] ROBERT J. GORMLEY, MICHAEL F. ZAROCHAK, PAUL W. DEFFENBAUGH, et al. Effect of initial wax medium on the Fischer-Tropsch slurry reaction[J]. Appl Catal A,1997,161:263−279. doi: 10.1016/S0926-860X(97)00077-X [25] CLAEYS M, VAN STEEN E, BOTHA T, et al. Oxidation of Hägg carbide during high-temperature Fischer–Tropsch synthesis: size-dependent thermodynamics and in situ observations[J]. ACS Catal,2021,11(22):13866−13879. doi: 10.1021/acscatal.1c03719 [26] ZHAO X Q, LIANG Y, HU Z Q, et al. Oxidation characteristics and magnetic properties of iron carbide and iron ultrafine particles[J]. J Appl Phys,1996,80(10):5857−5860. doi: 10.1063/1.363570 [27] THüNE P, MOODLEY P, SCHEIJEN F, et al. The effect of water on the stability of iron oxide and iron carbide nanoparticles in hydrogen and syngas followed by in situ X-ray absorption spectroscopy[J]. J Phys Chem C,2012,116(13):7367−7373. doi: 10.1021/jp210754k [28] KRISHNAMOORTHY S, LI A, IGLESIA E. Pathways for CO2 formation and conversion during Fishcher-Tropsch synthesis on iron-based catalysts[J]. Catal Lett,2002,80:77−86. doi: 10.1023/A:1015382811877 [29] CAO D-B, LI Y-W, WANG J, et al. Chain growth mechanism of Fischer-Tropsch synthesis on Fe5C2(001)[J]. J Mol Catal A,2011,346(1-2):55−69. doi: 10.1016/j.molcata.2011.06.009 [30] BING LIU, WENPING LI, JIAO ZHENG, et al. CO2 formation mechanism in Fischer-Tropsch synthesis over iron-based catalysts: A combined experimental and theoretical study[J]. Catal Sci Technol,2018,8:5288−5301. doi: 10.1039/C8CY01621F [31] BAI Y, LIU J, WANG T, et al. Theoretical study about adsorbed oxygen reduction over χ-Fe5C2: formation of H2O and CO2[J]. Mol Catal,2022,524:112236−112242. doi: 10.1016/j.mcat.2022.112236 [32] KRESSE G and FURTHMIILLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Comput Mater Sci,1996,6(1):15−50. doi: 10.1016/0927-0256(96)00008-0 [33] KRESSE G and FURTHMULLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Phys Rev B,1996,54(16):11169−11186. doi: 10.1103/PhysRevB.54.11169 [34] BLOCHL P E. Projector augmented-wave method[J]. Phys Rev B Condens Matter,1994,50(24):17953−17979. doi: 10.1103/PhysRevB.50.17953 [35] KRESSE G and JOUBERT D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Phys Rev B,1999,59(3):1758−1775. doi: 10.1103/PhysRevB.59.1758 [36] PERDEW J P, BURKE K and ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett,1996,77(18):3865−3868. doi: 10.1103/PhysRevLett.77.3865 [37] METHFESSEL M and PAXTON A T. High-precision sampling for Brillouin-zone integration in metals[J]. Phys Rev B Condens Matter,1989,40(6):3616−3621. doi: 10.1103/PhysRevB.40.3616 [38] HENKELMAN G and JóNSSON H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points[J]. J Chem Phys,2000,113(22):9978−9985. doi: 10.1063/1.1323224 [39] HENKELMAN G, UBERUAGA B P, JóNSSON H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths[J]. J Chem Phys,2000,113(22):9901−9904. doi: 10.1063/1.1329672 [40] SHEPPARD D, TERRELL R, HENKELMAN G. Optimization methods for finding minimum energy paths[J]. J Chem Phys,2008,128(13):134106. doi: 10.1063/1.2841941 [41] HENKELMAN G, JóNSSON H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives[J]. J Chem Phys,1999,111(15):7010−7022. doi: 10.1063/1.480097 [42] REUTER K, SCHEFFLER M. First-principles atomistic thermodynamics for oxidation catalysis: surface phase diagrams and catalytically interesting regions[J]. Phys Rev Lett,2003,90(4):46103−46106. doi: 10.1103/PhysRevLett.90.046103 [43] REUTER K, SCHEFFLER M. Composition, structure, and stability of RuO2(110) as a function of oxygen pressure[J]. Phys Rev B,2001,65(3):35406−35416. doi: 10.1103/PhysRevB.65.035406 [44] WANG T, TIAN X-X, LI Y-W, et al. Coverage-dependent CO adsorption and dissociation mechanisms on iron surfaces from DFT computations[J]. ACS Catal,2014,4(6):1991−2005. doi: 10.1021/cs500287r [45] HUO C-F, LI Y-W, WANG J, et al. Adsorption and dissociation of CO as well as CHx coupling and hydrogenation on the clean and oxygen pre-covered Co(0001) surfaces[J]. J Phys Chem C,2008,112:3840−3848. doi: 10.1021/jp710566t [46] WANG P, CHEN W, CHIANG F-K, et al. Synthesis of stable and low-CO2 selective epsilon-iron carbide Fischer-Tropsch catalysts[J]. Sci Adv,2018,4:2947−2953. doi: 10.1126/sciadv.aau2947 [47] WANG T, WANG S, LI Y-W, et al. Adsorption equilibria of CO coverage on β-Mo2C surfaces[J]. J Phys Chem C,2012,116(10):6340−6348. doi: 10.1021/jp300422g [48] https://janaf.nist.gov/. [49] CHIOU W. Structure and stability of Fe3C-cementite surfaces from first principles[J]. Surf Sci,2003,530(1-2):88−100. doi: 10.1016/S0039-6028(03)00352-2 [50] ZHAO S, LIU X-W, HUO C-F, et al. Determining surface structure and stability of ε-Fe2C, χ-Fe5C2, θ-Fe3C and Fe4C phases under carburization environment from combined DFT and atomistic thermodynamic studies[J]. Catal Struct React,2014,1(1):44−60. [51] BAI Y, LIU J, REN P, et al. Oxygen adsorption-induced morphological evolution of Hägg iron carbide at high oxygen chemical potentials[J]. J Phys Chem C,2021,125(5):3055−3065. doi: 10.1021/acs.jpcc.0c10822 [52] GAO W, GAO R, ZHAO Y, et al. Photo-driven syngas conversion to lower olefins over oxygen-decorated Fe5C2 Catalyst[J]. Chem,2018,4(12):2917−2928. doi: 10.1016/j.chempr.2018.09.017 [53] LIU S, TIAN X, WANG T, et al. Coverage dependent water dissociative adsorption on Fe(110) from DFT computation[J]. Phys Chem Chem Phys,2015,17(14):8811−21. doi: 10.1039/C5CP00044K -




 下载:
下载: