Combination of modified molybdenum sulfide catalyst and non-thermal plasma for syngas production from H2S-CO2 acid gas
-
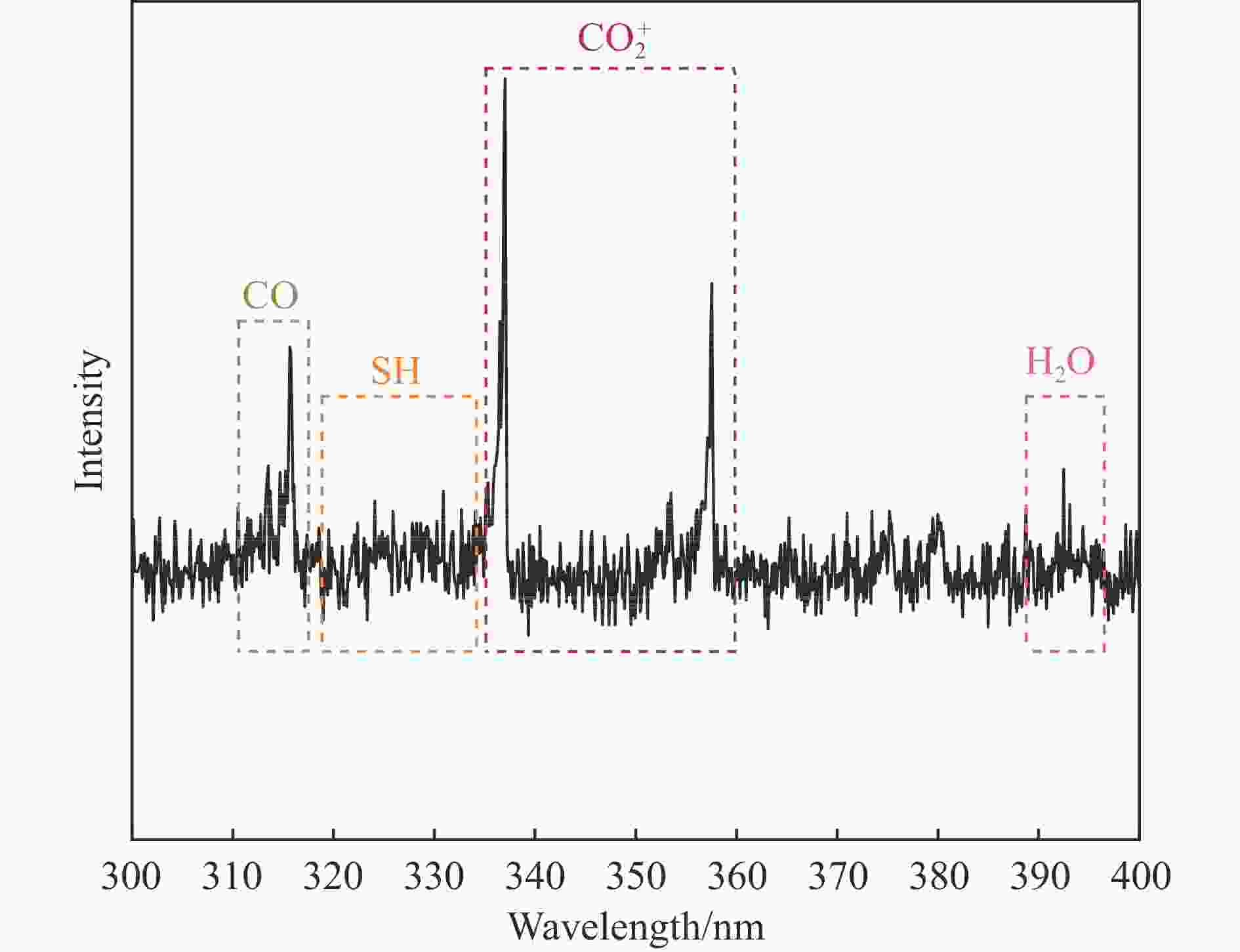
摘要: 以低温等离子体和催化剂耦合法将H2S和CO2混合酸气一步转化为合成气,既完成了两者清洁化处理,又实现了资源化利用,是一条制备合成气的新路线。本研究采用铜、锌为助剂对硫化钼催化剂改性,显著提升了其催化H2S-CO2制合成气反应性能。结合多种分析表征手段对比两种助剂引入后对硫化钼催化剂结构、组成、形貌、化合价态等物化特征的影响。通过控制低温等离子体放电条件,深入探究了两种助剂对低温等离子体下催化转化H2S和CO2酸气制合成气的反应性能影响规律和关键因素。研究发现,引入铜、锌助剂后,硫化钼活性相粒径减小且分散度高,提供了更多活性位点。同时也增强了对H2S和CO2分子吸附强度,从而更利于H2S和CO2分子的吸附活化,揭示出低温等离子体与改性硫化钼催化剂协同反应的构效关联。有关理论研究丰富拓展了低温等离子体-催化协同理论,并为改性硫化钼材料的合成提供借鉴。Abstract: The petrochemical, natural gas, and coal chemical industries will produce a large number of hydrogen sulfide (H2S) and carbon dioxide (CO2) mixed acid gas, causing serious damage to the environment and human health. At present, the most widely used treatment technology for H2S-containing mixed acid gas is the Claus process. Nevertheless, the Claus process is unable to achieve the recovery of hydrogen sources and the reduction of CO2 emissions, resulting in a considerable quantity of CO2 being discharged directly into the atmosphere, which has a detrimental impact on the global climate. Carbon, hydrogen, sulfur and other elements play an important role in the field of energy. Therefore, it is of great importance to explore new methods for the utilization of H2S and CO2 mixed acid gas to save energy, protect the environment and achieve green and low-carbon development. A non-thermal plasma-catalysis method is used to convert H2S and CO2 acid gas into syngas in a single step. This method achieves both the clean treatment of waste gas and its resource utilization, making it a novel route for syngas preparation. The non-thermal plasma contains high-energy electrons that can transfer energy to H2S and CO2 molecules in the form of inelastic collisions, thereby exciting them into free radicals, ions, excited molecules and atoms. Concurrently, the catalyst filled in the discharge gap can facilitate the chemical reactions of these active species. However, the stable molecular structure of H2S and CO2 presents a significant challenge to the improvement of energy efficiency, particularly in the context of high conversions of reactive molecule. The development of efficient catalysts is crucial to improve the H2S and CO2 conversion. The existing results demonstrate that electrons, photons and strong electric field generated by non-thermal plasma can be used to excite MoS2 catalyst to generate highly active electron-hole pairs. These in turn catalyze the conversion of H2S and CO2. This study used copper and zinc as promoters to modify the molybdenum sulfide catalyst, and the catalytic performance for the conversion of H2S-CO2 to syngas was effectively improved. A detailed comparison was made between the effects of the two promoters on the structure, composition, morphology, valence state, and other physicochemical characteristics of the molybdenum sulfide catalyst using various characterization methods. Furthermore, the influence factors of two types of promoters on the catalytic H2S-CO2 conversion was investigated by controlling the discharge conditions. The introduction of copper and zinc promoters was found to result in a reduction in the particle size of the molybdenum sulfide active phase, accompanied by a high degree of dispersion, which in turn led to an increase in the number of active sites. Concurrently, the adsorption strength of H2S and CO2 molecules was enhanced, which was conducive to the adsorption and activation of H2S and CO2. It revealed the structure-activity relationship between the modified molybdenum sulfide catalyst and plasma synergistic reaction. In addition, the theoretical research has enriched and expanded the theory of non-thermal plasma-catalysis. It has also provided a reference for the synthesis of modified molybdenum sulfide materials.
-
Key words:
- hydrogen sulfide /
- carbon dioxide /
- syngas /
- modified MoS2 catalyst /
- non-thermal plasma
-
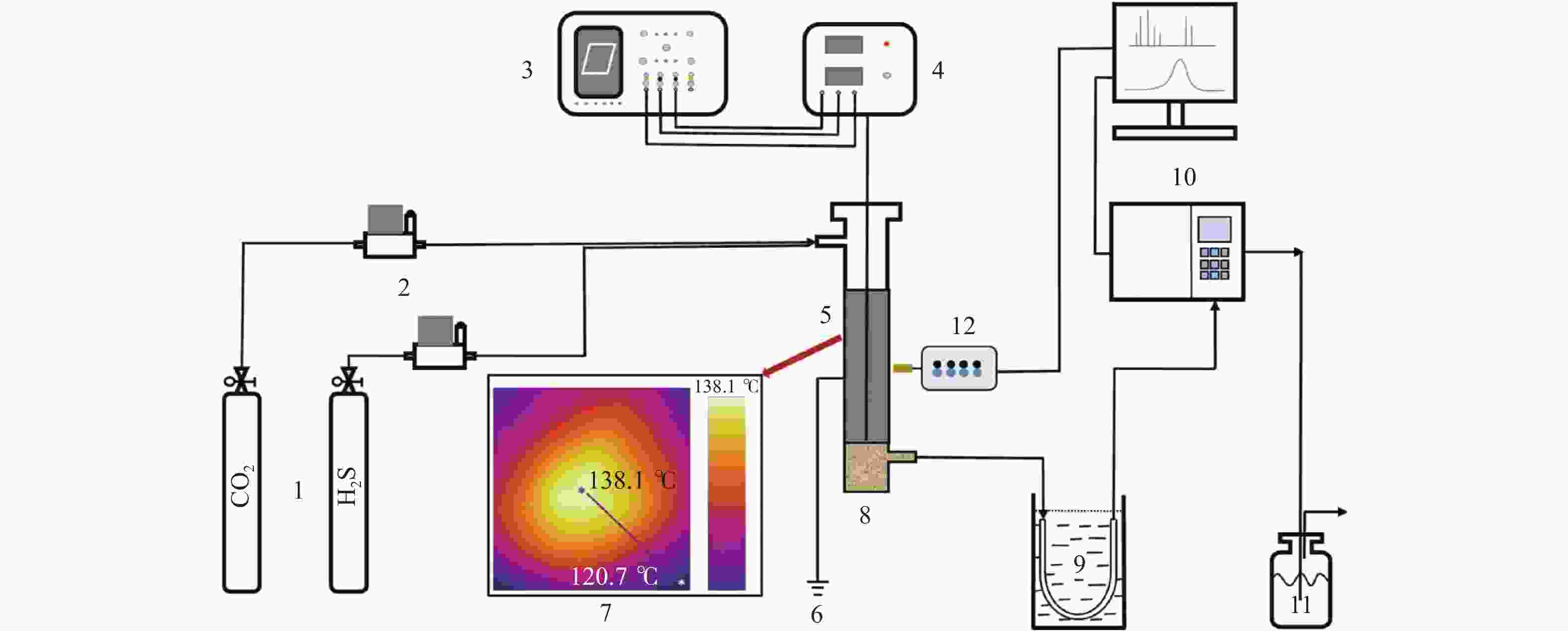
图 1 低温等离子体反应系统示意图
Figure 1 Schematic diagram of the non-thermal plasma experimental setup
1—Gas Cylinder; 2—Mass Flow Controller; 3—Oscilloscope; 4—High Voltage Power Supply; 5—DBD Reactor; 6—Grounding Electrode; 7—Temperature Distribution from Infrared Imaging Technology; 8—Sulphur Tank; 9—Cold Trap;10—Gas Chromatograph; 11—Lye Treatment; 12—OES Analysis.
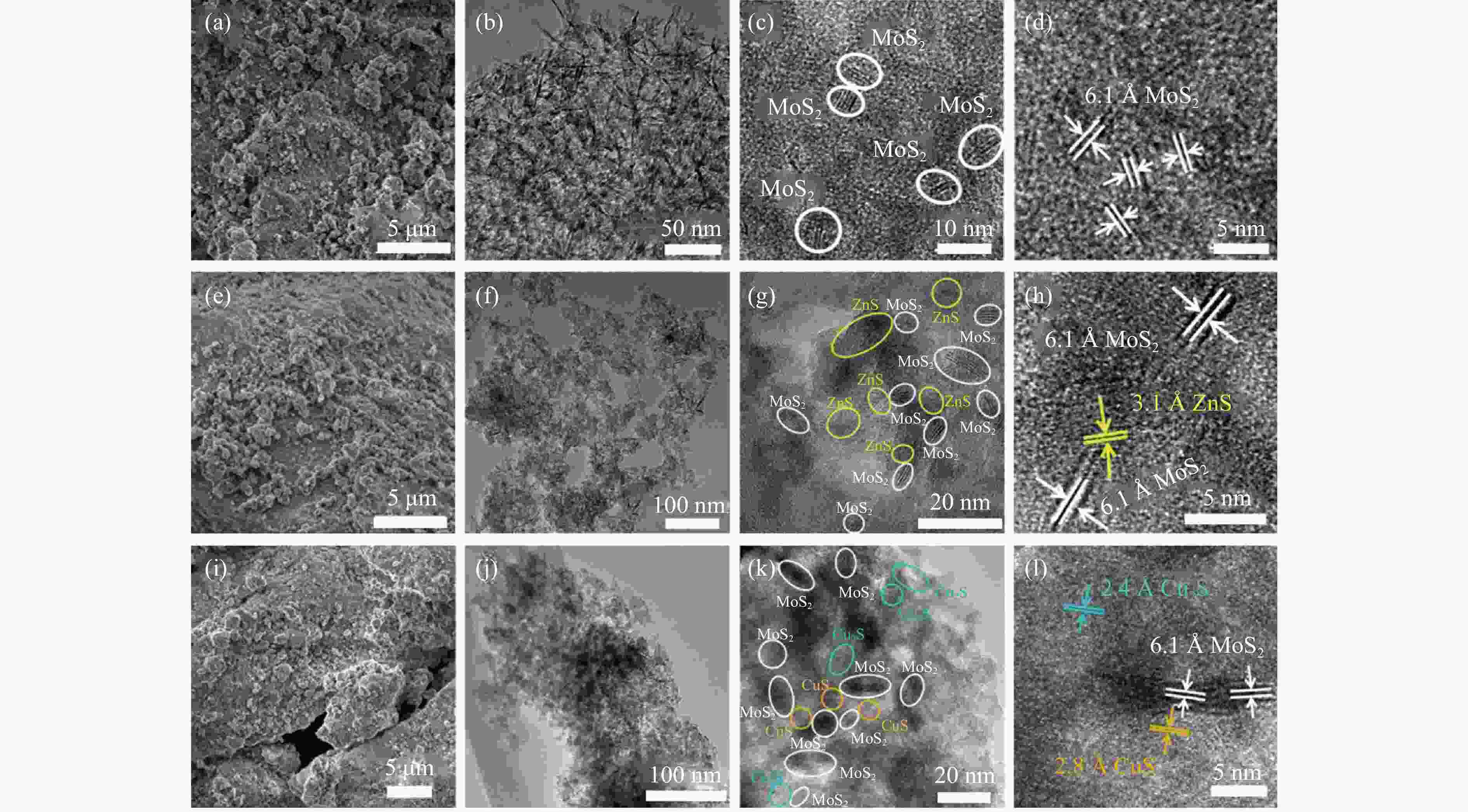
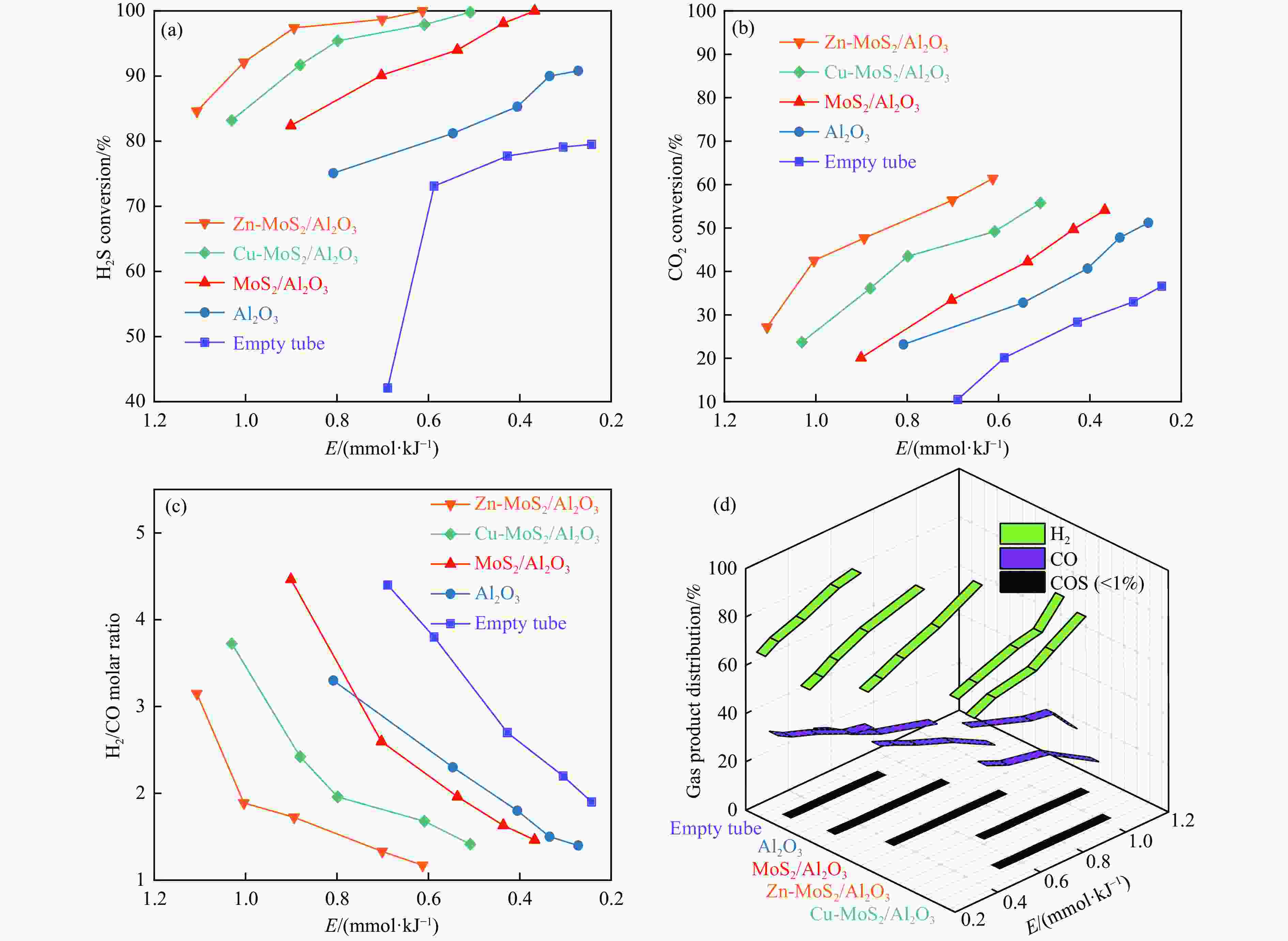
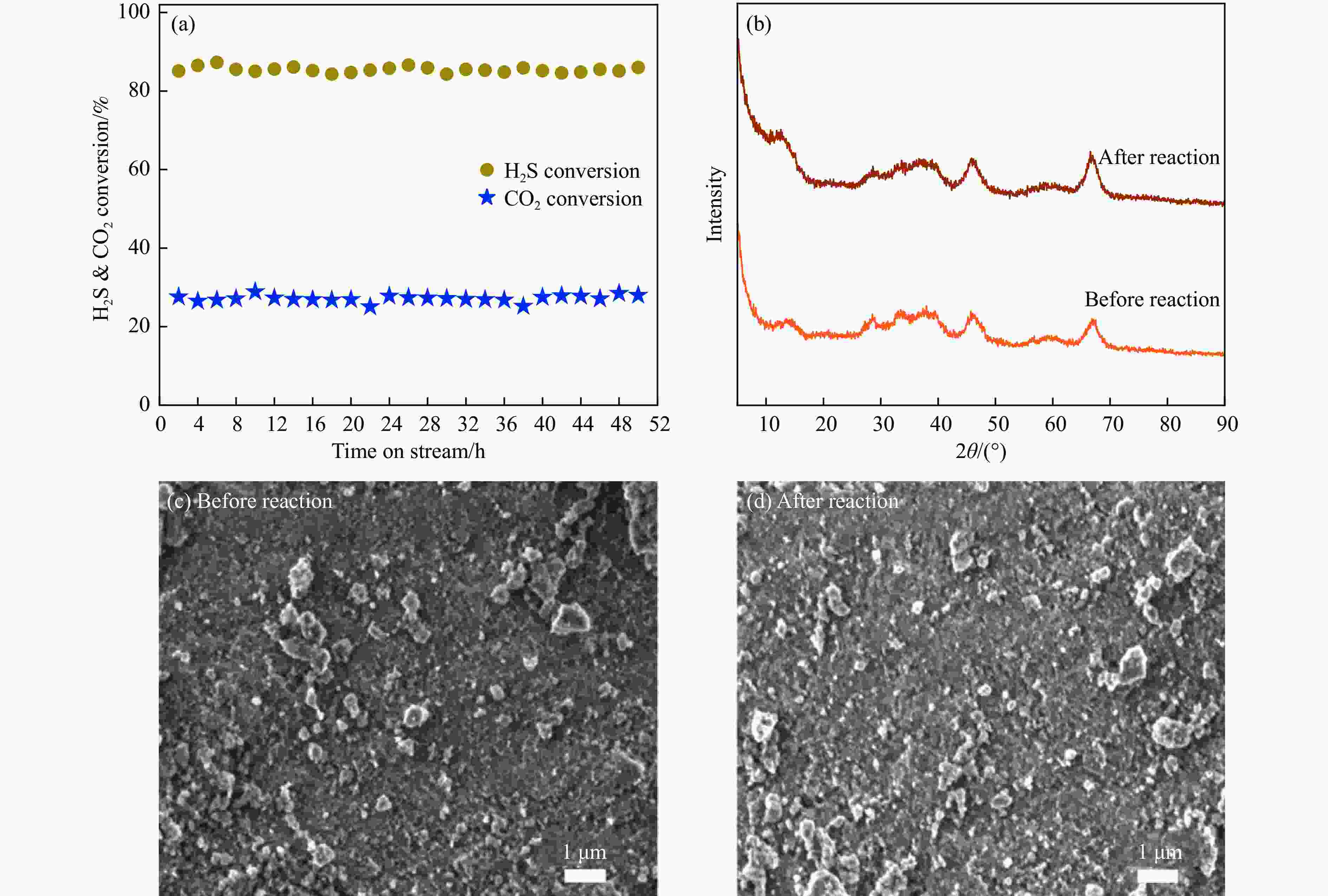
图 11 (a) Zn-MoS2/Al2O3 催化剂长周期测试; (b) Zn-MoS2/Al2O3催化剂反应前后XRD谱图; (c)和(d) Zn-MoS2/Al2O3催化剂反应前后SEM图像
Figure 11 (a) Long-time test of the Zn-MoS2/Al2O3 catalyst; (b) XRD patterns of the Zn-MoS2/Al2O3 catalyst before and after reaction; SEM images of the Zn-MoS2/Al2O3 catalyst before (c) and after (d) reactionReaction conditions: feed: H2S/CO2 molar ratio = 20:15; flow rate: 35 mL/min; catalyst bed volume: 15.0 mL; E: 1.1 mmol/kJ.
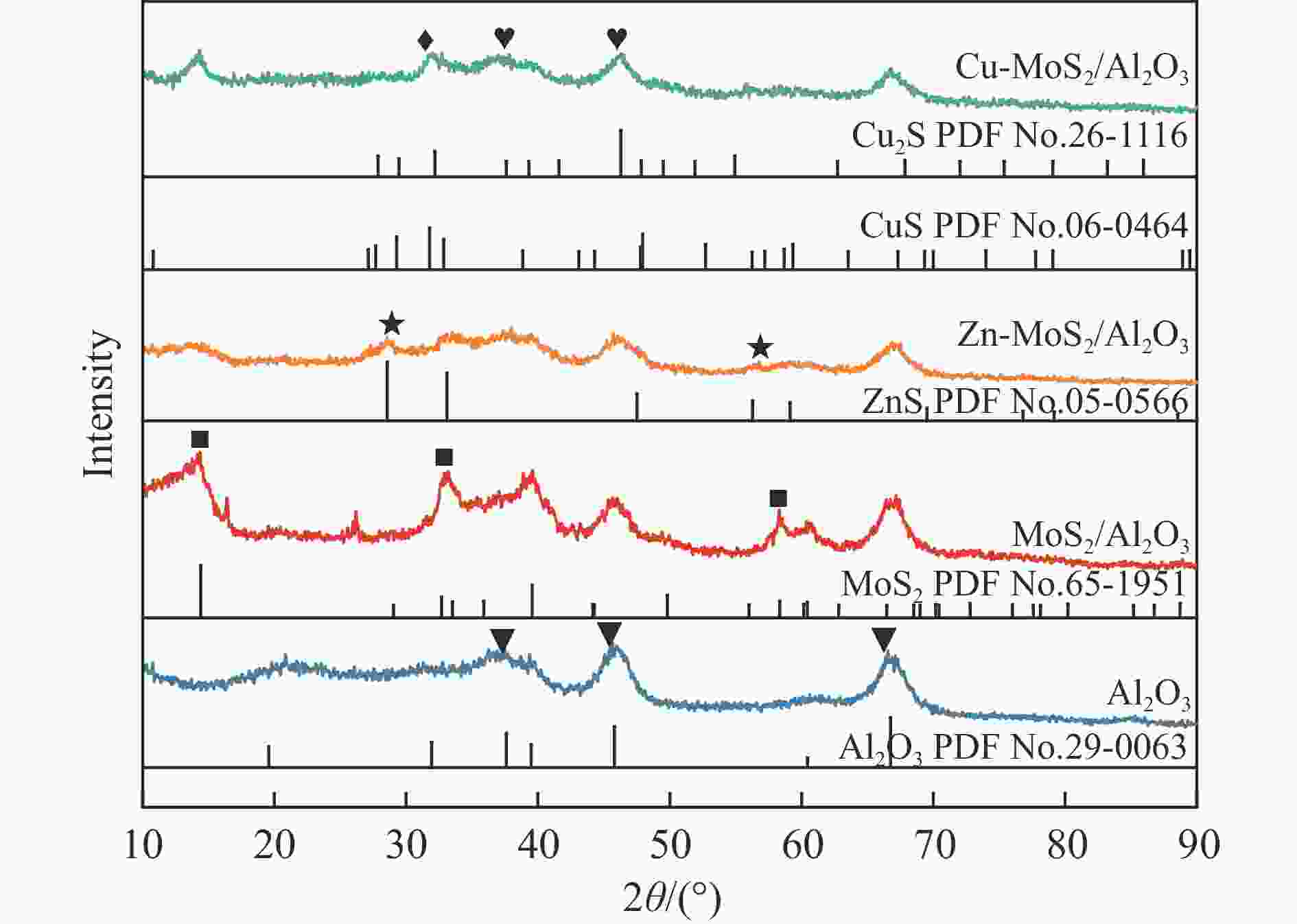
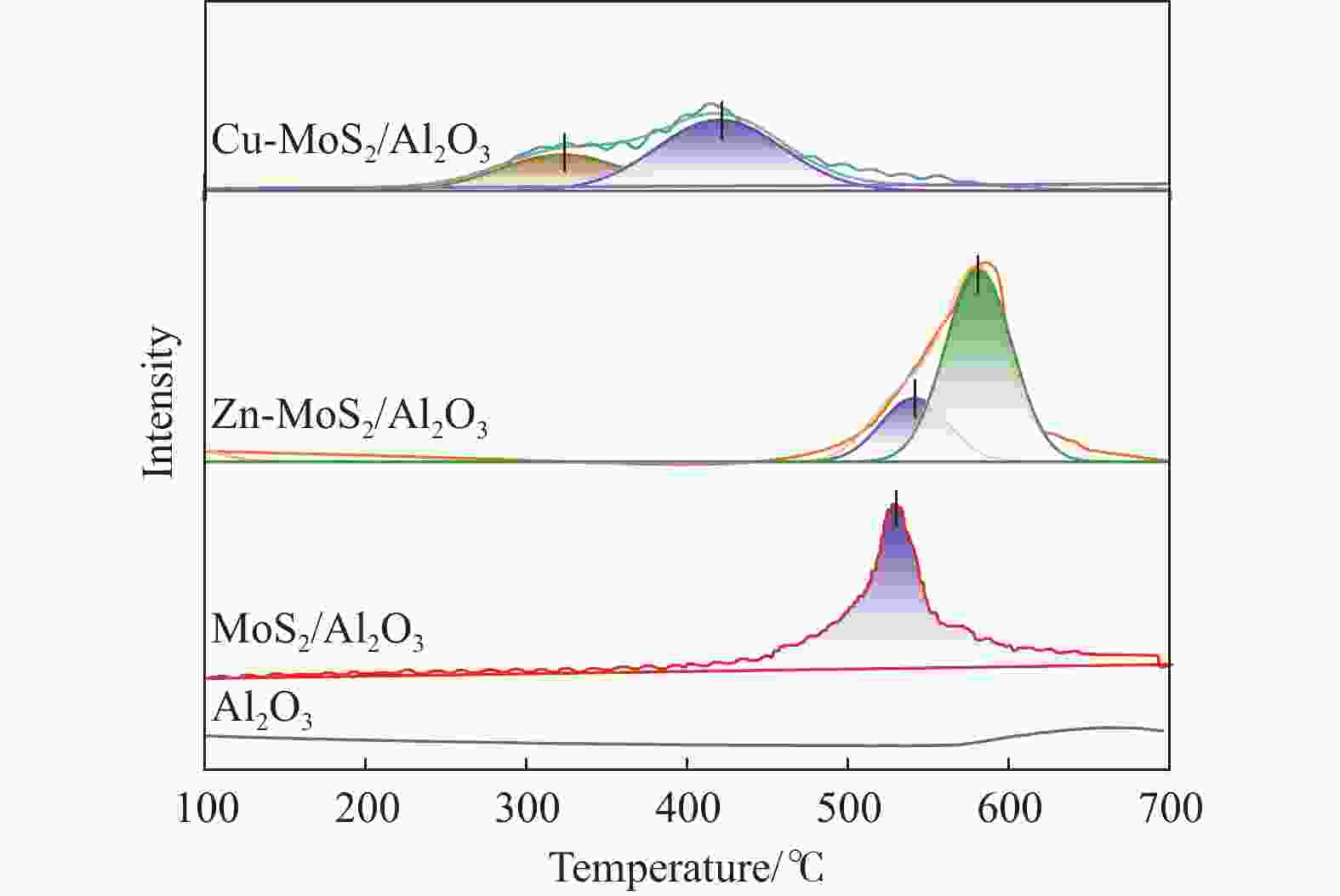
表 1 Cu-MoS2/Al2O3、Zn-MoS2/Al2O3和MoS2/Al2O3催化剂以及Al2O3载体理化性质
Table 1 Physic-chemical properties of the Cu-MoS2/Al2O3, Zn-MoS2/Al2O3, MoS2/Al2O3 catalysts and Al2O3 support
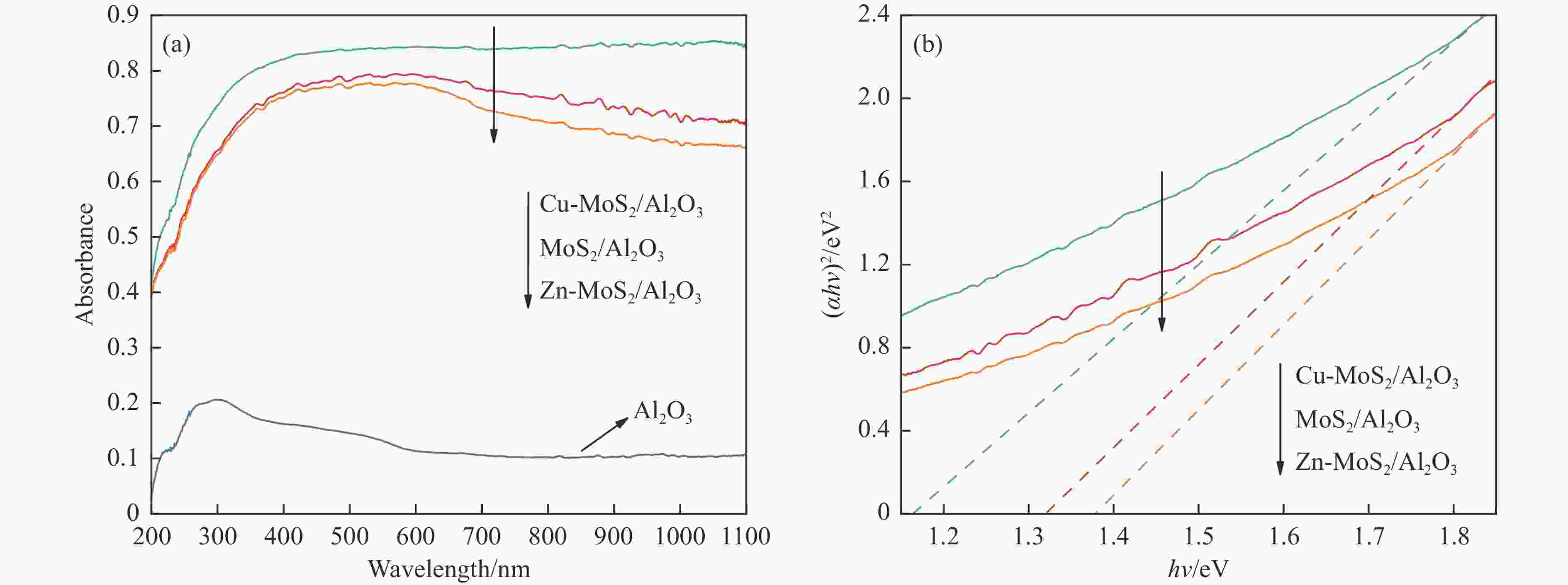
Sample Specific surface area/(m2·g−1) Particle size/(MoS2, nm) Lattice parameter a/(MoS2, nm) Band gap/eV Al2O3 300 − − − MoS2/Al2O3 252 8.8 0.316 1.32 Zn-MoS2/Al2O3 245 7.1 0.317 1.38 Cu-MoS2/Al2O3 259 7.5 0.319 1.16 -
[1] HALLIDAY C, HATTON T A. Sorbents for the capture of CO2 and other acid gases: A review[J]. Ind Eng Chem Res,2021,60(26):9313−9346. doi: 10.1021/acs.iecr.1c00597 [2] GUPTA A K, IBRAHIM S, Al SHOAIBI A. Advances in sulfur chemistry for treatment of acid gases[J]. Prog Energy Combust,2016,54:65−92. doi: 10.1016/j.pecs.2015.11.001 [3] 喻昕蕾, 潘伟童, 高瑞, 等. LaCoO3对H2S选择氧化性能的影响[J]. 燃料化学学报,2019,47(8):973−979.YU Xinlei, PAN Weitong, GAO Rui, et al. Selective oxidation of H2S over the LaCoO3 catalyst[J]. J Fuel Chem Technol,2019,47(8):973−979. [4] 王晗, 樊升, 王森, 等. 二氧化碳加氢制一些烃类化合物的研究进展[J]. 燃料化学学报,2021,49(11):1609−1619. doi: 10.1016/S1872-5813(21)60122-6WANG Han, FAN Sheng, WANG Sen, et al. Research progresses in the hydrogenation of carbon dioxide to certain hydrocarbon products[J]. J Fuel Chem Technol,2021,49(11):1609−1619. doi: 10.1016/S1872-5813(21)60122-6 [5] LAGAS J A, BORSBOOM J, BERBEN P H. Selective-oxidation catalyst improves Claus process[J]. Oil Gas J,1988,86:67−71. [6] ZHENG X, LEI G, WANG S, et al. Advances in resources recovery of H2S: A review of desulfurization processes and catalysts[J]. ACS Catal,2023,13(17):11723−11752. doi: 10.1021/acscatal.3c02294 [7] SU H, LI Y, LI P, et al. Simultaneous recovery of carbon and sulfur resources from reduction of CO2 with H2S using catalysts[J]. J Energy Chem,2016,25(1):110−116. doi: 10.1016/j.jechem.2015.08.009 [8] MA W, WANG H, YU W, et al. Achieving simultaneous CO2 and H2S conversion via a coupled solar-driven electrochemical approach on non-precious-metal catalysts[J]. Angew Chem Int Ed,2018,57(13):3473−3477. doi: 10.1002/anie.201713029 [9] ZHANG F, WEI Z, JIANG G, et al. Synergistic conversion of acid gases (H2S and CO2) to valuable chemicals: Carbonyl sulfide synthesis over vacancy-defective CoMo sulfide catalysts[J]. Appl Catal B: Environ,2022,319:121912. doi: 10.1016/j.apcatb.2022.121912 [10] ZHOU Q, WU P, LIU C, et al. Highly selective conversion of H2S/CO2 and reaction mechanism with CeO2 loading of MgO as catalysts[J]. Ind Eng Chem Res,2023,62(17):6660−6671. doi: 10.1021/acs.iecr.3c00257 [11] BASSANI A, BOZZANO G, PIROLA C, et al. Low impact methanol production from sulfur rich coal gasification[J]. Energy Procedia,2017,105:4519−4524. doi: 10.1016/j.egypro.2017.03.970 [12] WU J, HE R, CHENG S, et al. Simultaneous immobilization of CO2 and H2S by propargyl amines under mild conditions: Efficient synthesis of thiazolidine-2-ones[J]. ACS Sustainable Chem Eng,2022,10(3):1214−1219. doi: 10.1021/acssuschemeng.1c07010 [13] ZHAO L, LIU X, MU X, et al. Highly selective conversion of H2S-CO2 to syngas by combination of non-thermal plasma and MoS2/Al2O3[J]. J CO2 Util,2020,37:45−54. doi: 10.1016/j.jcou.2019.11.021 [14] 余康, 李民, 孙高攀, 等. 介质阻挡放电等离子体转化H2S-CO2酸气制合成气的影响因素研究[J]. 燃料化学学报(中英文),2023,51(12):1782−1790. doi: 10.1016/S1872-5813(23)60365-2YU Kang, LI Min, SUN Gaopan, et al. The influence factors of dielectric barrier discharge plasma to production of syngas derived from H2S-CO2 acid gas[J]. J Fuel Chem Technol,2023,51(12):1782−1790. doi: 10.1016/S1872-5813(23)60365-2 [15] ZHAO L, WANG Y, LI X, et al. Hydrogen production via decomposition of hydrogen sulfide by synergy of non-thermal plasma and semiconductor catalysis[J]. Int J Hydrogen Energy,2013,38(34):14415−14423. doi: 10.1016/j.ijhydene.2013.09.008 [16] MEI D, LIU S, YANIK J, et al. Plasma-catalytic reforming of naphthalene and toluene as biomass tar over honeycomb catalysts in a gliding arc reactor[J]. ACS Sustainable Chem Eng,2022,10(27):8958−8969. doi: 10.1021/acssuschemeng.2c02495 [17] FONSECA H A B, VERGA L G, DA SILVA J L F. Theoretical tuning of the Cu/S ratio on two-dimensional CuSx materials for the CO2 electrochemical reduction[J]. J Phys Chem C,2023,127(50):24118−24128. doi: 10.1021/acs.jpcc.3c05682 [18] SHI X, WANG L, DAI W, et al. CO2 photoreduction catalyzed by Cu-deficient Cu1.95S@CuS: Enhanced performance via boosted directional interfacial charge transfer[J]. ACS Catal,2023,13(8):5264−5271. doi: 10.1021/acscatal.3c00492 [19] ZHAO L, WANG Y, WANG A, et al. Cr-doped ZnS semiconductor catalyst with high catalytic activity for hydrogen production from hydrogen sulfide in non-thermal plasma[J]. Catal Today,2019,337:83−89. doi: 10.1016/j.cattod.2019.02.032 [20] ZHAO L, WANG Y, SUN Z, et al. Synthesis of highly dispersed metal sulfide catalysts via low temperature sulfidation in dielectric barrier discharge plasma[J]. Green Chem,2014,16(5):2619−2626. doi: 10.1039/C3GC42313A [21] LASHGARI M, GHANIMATI M. Photocatalytic degradation of H2S aqueous media using sulfide nanostructured solid-solution solar-energy-materials to produce hydrogen fuel[J]. J Hazard Mater,2018,345:10−17. doi: 10.1016/j.jhazmat.2017.10.062 [22] MA G, YAN H, ZONG X, et al. Photocatalytic splitting of H2S to produce hydrogen by gas-solid phase reaction[J]. Chin J Catal,2008,29(4):313−315. doi: 10.1016/S1872-2067(08)60029-7 [23] 李莹, 赵璐, 刘晓展, 等. 低温等离子体制备低碳醇合成用KNiMo基催化剂及其结构性能表征[J]. 燃料化学学报, 2019, 47 (5): 513−522.LI Ying, ZHAO Lu, LIU Xiaozhan, et al. Preparation of KNiMo-based catalysts by using non-thermal plasma and their catalytic performance in the synthesis of higher alcohols from syngas[J]. J Fuel Chem Technol, 2019, 47 (5): 513−521.) [24] 王乾浩, 赵璐, 孙付琳, 等. ZSM-5催化剂与低温等离子体协同转化H2S-CO2制合成气[J]. 化工学报,2022,73(1):255−265.WANG Qianhao, ZHAO Lu, SUN Fulin, et al. Production of syngas derived from H2S-CO2 via synergy of ZSM-5 catalyst and non-thermal plasma[J]. CIESC J,2022,73(1):255−265. [25] KIM H H, LEE Y H, OGATA A, et al. Plasma-driven catalyst processing packed with photocatalyst for gas-phase benzene decomposition[J]. Catal Commun,2003,4(7):347−351. doi: 10.1016/S1566-7367(03)00086-4 [26] TU X, WHITEHEAD J C. Plasma dry reforming of methane in an atmospheric pressure AC gliding arc discharge: Co-generation of syngas and carbon nanomaterials[J]. Int J Hydrog Energy,2014,39(18):9658−9669. doi: 10.1016/j.ijhydene.2014.04.073 [27] CULLITY B D. Elements of X-ray[J]. by M. Cohen, Addison-Weslay, Boston, 1978: 447−478. [28] HU J, YU L, DENG J, et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol[J]. Nat Catal,2021,4(3):242−250. doi: 10.1038/s41929-021-00584-3 [29] MAO J, LIU H, CUI X, et al. Direct conversion of methane with O2 at room temperature over edge-rich MoS2[J]. Nat Catal,2023,6(11):1052−1061. doi: 10.1038/s41929-023-01030-2 [30] 赵立业, 李恒, 王亮, 等. 卤素原子对卤氧化铋(BiOX, X = Cl, Br, I)光催化性能的影响[J]. 燃料化学学报(中英文),2022,50(1):122−128.ZHAO Liye, LI Heng, WANG Liang, et al. Effect of halogen atoms on photocatalytic activity of bismuth oxyhalide (BIOX, X = Cl, Br, I)[J]. J Fuel Chem Technol,2022,50(1):122−128. [31] FRIDMAN A. Plasma Chemistry[M]. Cambridge University Press, 2008. [32] RIAD M, MIKHAIL S. Effect of support modification on the characterization and catalytic activity of Mo/Al2O3 catalysts[J]. J Energy Chem,2015,24(4):520−528. doi: 10.1016/j.jechem.2015.06.003 [33] LIANG M, KANG W, XIE K. Comparison of reduction behavior of Fe2O3, ZnO and ZnFe2O4 by TPR technique[J]. J Nat Gas Chem,2009,18(1):110−113. doi: 10.1016/S1003-9953(08)60073-0 [34] LUO M F, FANG P, HE M, et al. In situ XRD, Raman, and TPR studies of CuO/Al2O3 catalysts for CO oxidation[J]. J Mol Catal A-Chem,2005,239(1-2):243−248. doi: 10.1016/j.molcata.2005.06.029 [35] LIU B S, ZHANG Y, LIU J F, et al. Characteristic and mechanism of methane dehydroaromatization over Zn-based/HZSM-5 catalysts under conditions of atmospheric pressure and supersonic jet expansion[J]. J Phys Chem C,2011,115(34):16954−16962. doi: 10.1021/jp2027065 [36] FANG X, YAO S, QING Z, et al. Study on silica supported CuCrMo nitrobenzene hydrogenation catalysts[J]. Appl Catal A: Gen,1997,161(1-2):129−135. doi: 10.1016/S0926-860X(97)00038-0 [37] SANTOS R C R, BRAGA D M V, PINHEIRO A N, et al. Role of Cu, Ni and Co metals in the acidic and redox properties of Mo catalysts supported on Al2O3 spheres for glycerol conversion[J]. Catal Sci Technol,2016,6(13):4986−5002. doi: 10.1039/C6CY00096G [38] WANG C H, WENG H S. Promoting effect of molybdenum on CuO/γ-Al2O3 catalyst for the oxidative decomposition of (CH3)2S2[J]. Appl Catal A: Gen,1998,170(1):73−80. doi: 10.1016/S0926-860X(98)00050-7 [39] NAVARRO R M, ÁLVAREZ-GALVÁN M C, ROSA F, et al. Hydrogen production by oxidative reforming of hexadecane over Ni and Pt catalysts supported on Ce/La-doped Al2O3[J]. Appl Catal A: Gen,2006,297(1):60−72. doi: 10.1016/j.apcata.2005.08.036 [40] 卢诗文, 吴康, 刘鹏, 等. 钴酸镧基钙钛矿耦合非热等离子体催化转化CO2的实验研究[J]. 环境科学学报, 2023, 43 (2): 424−432.LU Shiwen, WU Kang, LIU Peng, et al. Experimental study on catalytic CO2 conversion by LaCoO3-based perovskites coupled with non-thermal plasma[J] Acta Scien Circum, 2023, 43 (2): 424−432.) [41] ZHU Y, YUK S F, ZHENG J, et al. Environment of metal-O-Fe bonds enabling high activity in CO2 reduction on single metal atoms and on supported nanoparticles[J]. J Am Chem Soc,2021,143(14):5540−5549. doi: 10.1021/jacs.1c02276 [42] HUANG W, LIU F, HUANG Y, et al. Facile one-pot synthesis of hollow-structured CuS/Cu2S hybrid for enhanced electrochemical determination of glucose[J]. Electrochemistry,2021,89(4):340−347. doi: 10.5796/electrochemistry.21-00027 [43] LIU X, ZHAO L, LI Y, et al. Ni-Mo sulfide semiconductor catalyst with high catalytic activity for one-step conversion of CO2 and H2S to syngas in non-thermal plasma[J]. Catalysts,2019,9(6):525. doi: 10.3390/catal9060525 [44] ZHAO L, LI Y, LIU X, et al. Low-temperature synthesis of high-performance nano-MoS2-based catalyst via non-thermal plasma for higher alcohol synthesis from syngas[J]. Catal Today,2020,355:17−25. doi: 10.1016/j.cattod.2019.01.069 [45] AHMADI M, ALAVI S M, LARIMI A. Highly active platinum decorated BiVO4 nanosheet/TiO2 nanobelt heterojunction for photocatalytic CO2 reduction[J]. Surf Interfaces,2024,45:103908. doi: 10.1016/j.surfin.2024.103908 [46] ZHAO G B, JOHN S, ZHANG J J, et al. Production of hydrogen and sulfur from hydrogen sulfide in a nonthermal-plasma pulsed corona discharge reactor[J]. Chem Eng Sci,2007,62(8):2216−2227. doi: 10.1016/j.ces.2006.12.052 [47] RAO M U, BHARGAVI K, MADRAS G, et al. Basic metal oxide integrated DBD packed bed reactor for the decomposition of CO2[J]. Chem Eng J,2023,468:143671. doi: 10.1016/j.cej.2023.143671 [48] STEEN M L, BUTOI C I, FISHER E R. Identification of gas-phase reactive species and chemical mechanisms occurring at plasma-polymer surface interfaces[J]. Langmuir,2001,17(26):8156−8166. doi: 10.1021/la0106642 [49] THOMAS L, MAILLÉ L, BADIE J M, et al. Microwave plasma chemical vapour deposition of tetramethylsilane: Correlations between optical emission spectroscopy and film characteristics[J]. Surf Coat Technol,2001,142:314−320. -




 下载:
下载: