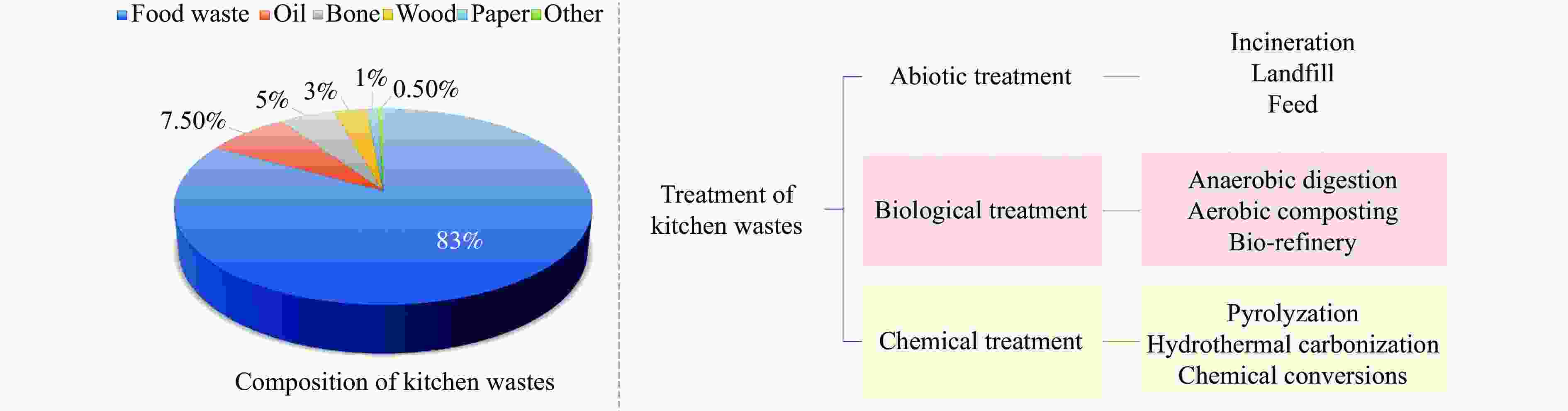
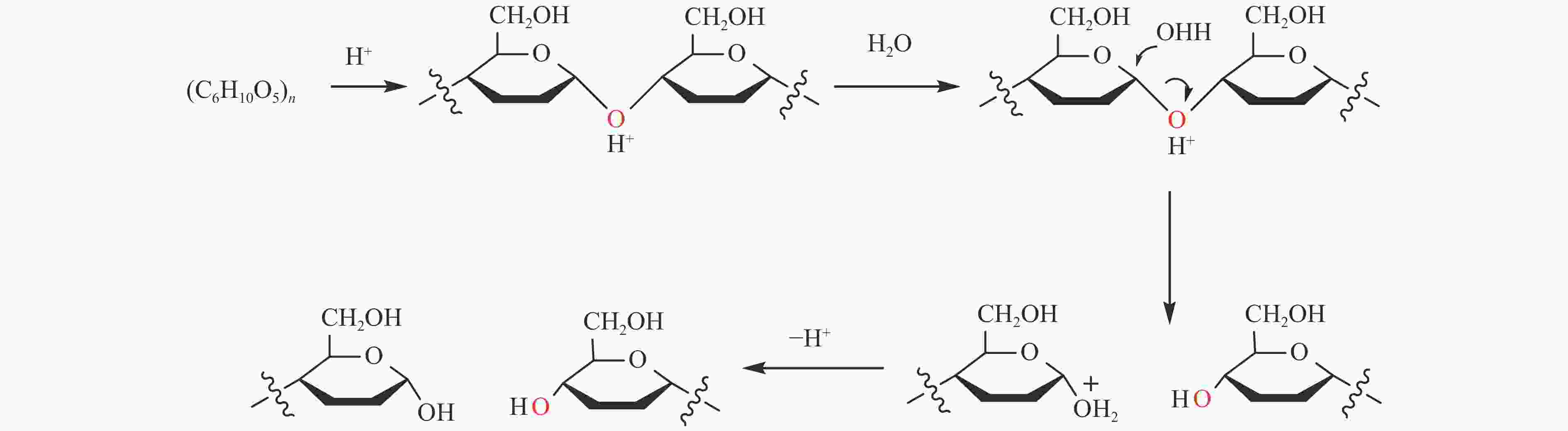
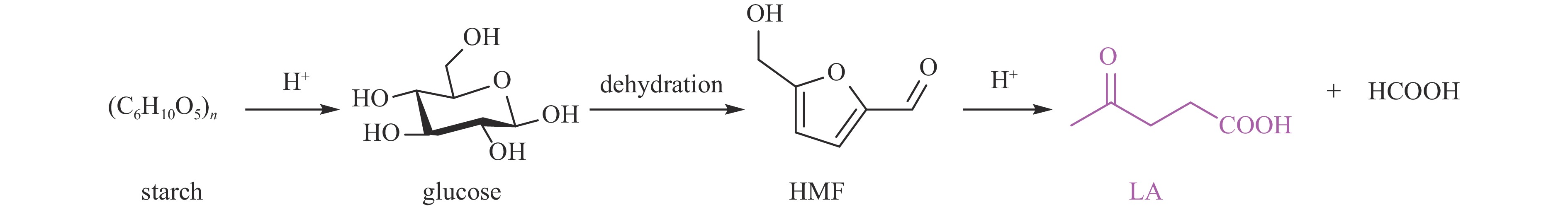
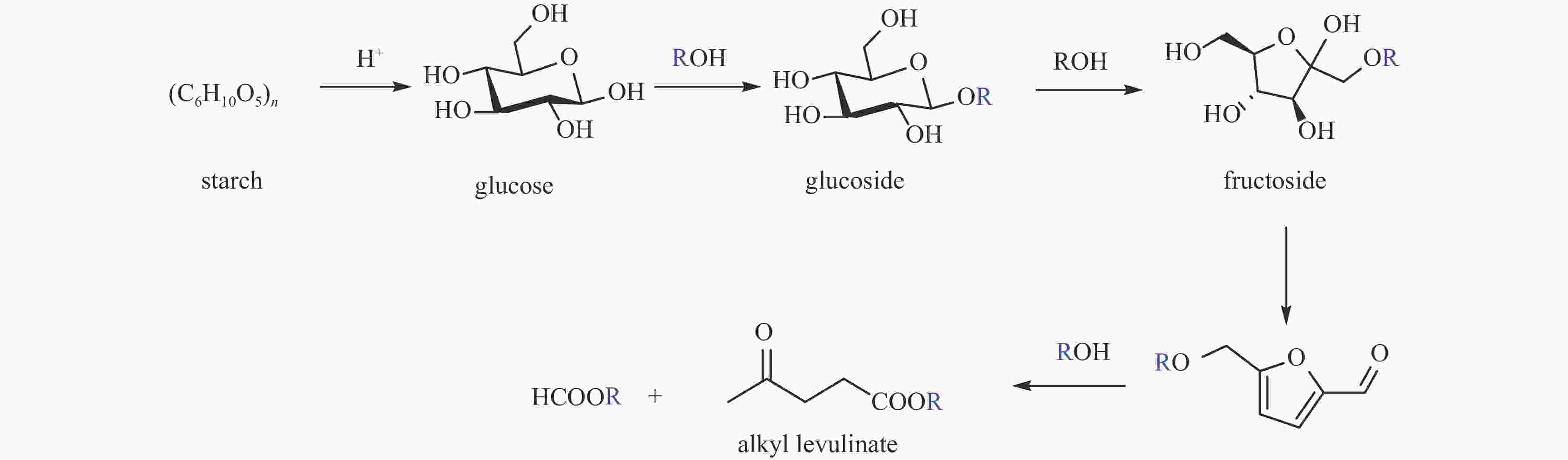
| [1] |
潘翔, 张铭, 张焦, 等. 餐厨垃圾厌氧处理技术研究进展[J]. 广东化工,2024,51(5):114−115+113. doi: 10.3969/j.issn.1007-1865.2024.05.033.PANG Xiang, ZHANG Ming, ZHANG Jiao, et al. Research progress of food waste anaerobic digestion technology[J]. Guangdong Chemical Industry,2024,51(5):114−115+113. doi: 10.3969/j.issn.1007-1865.2024.05.033.
|
| [2] |
辛梓弘. 餐厨垃圾处理技术研究[J]. 当代化工研究,2022,09:63−65. doi: 10.3969/j.issn.1672-8114.2022.06.021XIN Zihong. Study on food waste treatment technologies[J]. Modern Chemical Research,2022,09:63−65. doi: 10.3969/j.issn.1672-8114.2022.06.021
|
| [3] |
邓松圣, 冷夕杜, 戴飞. 餐厨垃圾处理的恶臭气体产生与控制对策探讨[J]. 资源节约与环保,2022,03:77−80. doi: 10.3969/j.issn.1673-2251.2022.10.020DENG Songsheng, LENG Xidu, DAI Fei. Discussion on the generation and control measures of odorous gases in food waste treatment[J]. Resources Economization & Environmental Protection,2022,03:77−80. doi: 10.3969/j.issn.1673-2251.2022.10.020
|
| [4] |
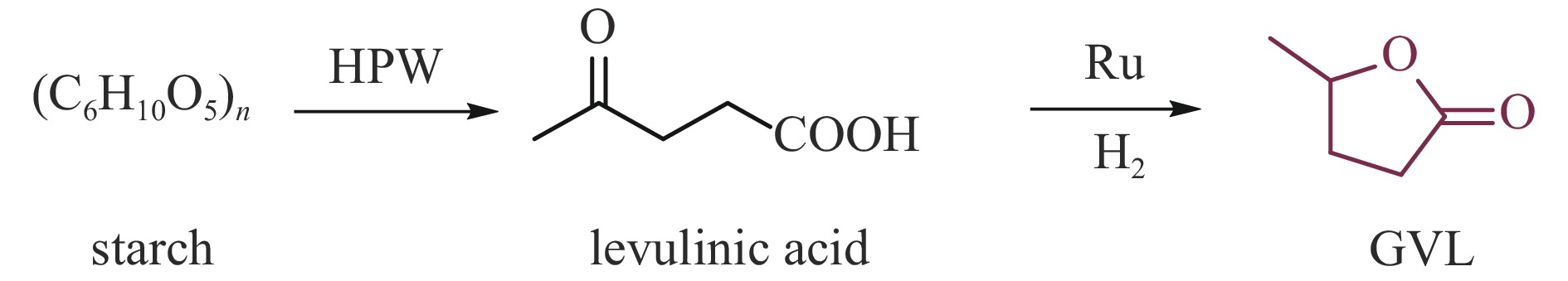
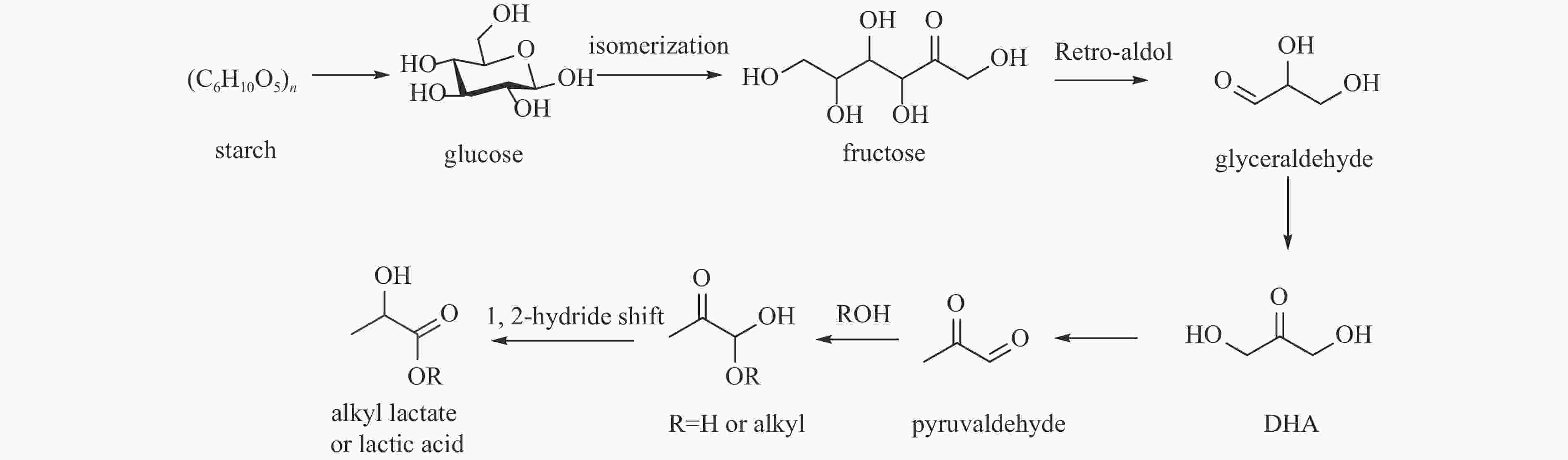
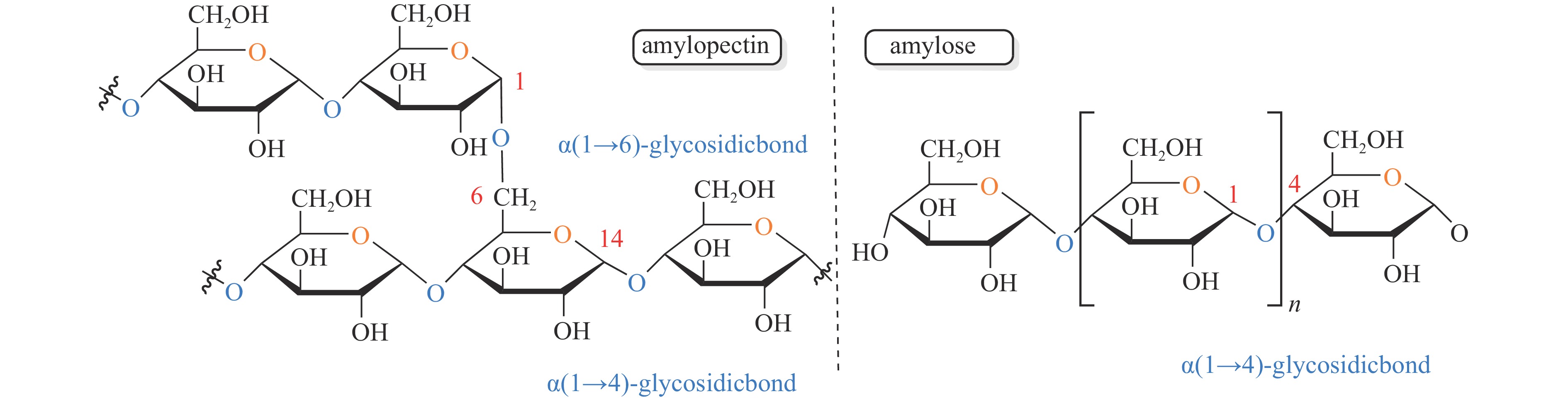
KUMAR V B, PULIDINDI I N, GEDANKEN A. Selective conversion of starch to glucose using carbon based solid acid catalyst[J]. Renew Energy,2015,78:141−145. doi: 10.1016/j.renene.2014.12.070
|
| [5] |
崔文静, 陆敏博. 餐厨垃圾处理现状及今后发展趋势[J]. 广东化工,2021,48(19):140−141. doi: 10.3969/j.issn.1007-1865.2021.19.066CUI Wenjing, LU Minbo. The present disposing situation and development trend of kitchen waste[J]. Guangdong Chemical Industry,2021,48(19):140−141. doi: 10.3969/j.issn.1007-1865.2021.19.066
|
| [6] |
易志刚. 餐厨垃圾收运与资源化利用研究进展[J]. 中国资源综合利用,2021,39(12):116−119+125. doi: 10.3969/j.issn.1008-9500.2021.12.032YI Zhigang. Research progress on the collection, transportation and resource utilization of kitchen waste[J]. China Resources Comprehensive Utilization,2021,39(12):116−119+125. doi: 10.3969/j.issn.1008-9500.2021.12.032
|
| [7] |
周俊, 王梦瑶, 王改红, 等. 餐厨垃圾资源化利用技术研究现状及展望[J]. 生物资源,2020,42(1):87−96.ZHOU Jun, WANG Mengyao, WANG gaihong, et al. Research status and prospect of food waste utilization technology[J]. Biotic Resources,2020,42(1):87−96.
|
| [8] |
王丽华, 李宇宸, 韩聪. 城市餐厨垃圾处理技术分析及思路分析[J]. 中国资源综合利用,2018,12(36):73−75. doi: 10.3969/j.issn.1008-9500.2018.12.022WANG Lihua, LIU Yuchen, HAN Cong. Technical analysis and thinking analysis of urban kitchen waste treatment technology[J]. China Resources Comprehensive Utilization,2018,12(36):73−75. doi: 10.3969/j.issn.1008-9500.2018.12.022
|
| [9] |
陈必鸣. 餐厨垃圾预处理技术综述[J]. 环境卫生工程,2015,23(5):10−12. doi: 10.3969/j.issn.1005-8206.2015.05.004CHEN Biming. Food waste pretreatment technologies[J]. Environmental Sanitation Engineering,2015,23(5):10−12. doi: 10.3969/j.issn.1005-8206.2015.05.004
|
| [10] |
李游, 刘喜, 赵石铁, 等. 餐厨垃圾资源化处理预处理方案对比分析[J]. 环境与发展,2019,31(11):218−219+222.LI You, LIU Xi, ZHAO Shitie, et al. Comparative analysis of the pretreatment schemes for recycling treatment of food waste[J]. Environment and Development,2019,31(11):218−219+222.
|
| [11] |
HUANG Y B, Fu Y. Hydrolysis of cellulose to glucose by solid acid catalysts[J]. Green Chem,2013,15:1095−1111. doi: 10.1039/c3gc40136g
|
| [12] |
ONDA A. Selective hydrolysis of cellulose and polysaccharides into sugars by catalytic hydrothermal method using sulfonated activated-carbon[J]. J Jpn Pet Inst,2012,55(2):73−86. doi: 10.1627/jpi.55.73
|
| [13] |
RAJKUMAR T, RAO G R. Porous hydrous zirconia supported 12-tungstophosphoric acid catalysts for liquid-phase esterification of 2-ethyl-1-hexanol[J]. J Mol Catal A Chem,2008,295:1−9. doi: 10.1016/j.molcata.2008.08.008
|
| [14] |
ZHU S, LI J, CHENG F, et al. Forming a Cu-based catalyst for efficient hydrogenation conversion of starch into glucose[J]. Catalysts,2024,14:132. doi: 10.3390/catal14020132
|
| [15] |
KUMAR V B, PULIDINI A, Indra N, GEDANKEN A. Glucose production from potato peel waste under microwave irradiatio[J]. J Mol Catal A Chem,2016,417:163−167. doi: 10.1016/j.molcata.2016.03.025
|
| [16] |
YU I K M, TSANG D C W, YIP A C K, et al. Valorization of food waste into hydroxymethylfurfural: Dual role of metal ions in successive conversion steps[J]. Bioresource Technol,2016,219:338−347. doi: 10.1016/j.biortech.2016.08.002
|
| [17] |
BOZELL J J, PETERSEN G R. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited[J]. Green Chem,2010,12:539−554. doi: 10.1039/b922014c
|
| [18] |
FAN W, VERRIER C, QUENEAU Y, et al. 5-Hydroxymethylfurfural (HMF) in organic synthesis: A review of its recent applications towards fine chemicals[J]. Curr Org Synth,2019,16:583−614. doi: 10.2174/1570179416666190412164738
|
| [19] |
MUKHERJEE A, DUMONT M J, RAGHAVAN V. Review: sustainable production of hydroxymethylfurfural and levulinic acid: challenges and opportunities[J]. Biomass Bioenergy,2015,72:143−183. doi: 10.1016/j.biombioe.2014.11.007
|
| [20] |
COVADONGA L T, ALMUDENA L, BEATRIZ C, et al. Microwave heating for the catalytic conversion of melon rind waste into biofuel precursors[J]. J Clean Prod,2016,138:59−69. doi: 10.1016/j.jclepro.2016.03.122
|
| [21] |
YU I K M, TSANG D C W, CHEN S S, et al. Polar aprotic solvent-water mixture as the medium for catalytic production of hydroxymethylfurfural (HMF) from bread waste[J]. Bioresource Technol,2017,245:456−462. doi: 10.1016/j.biortech.2017.08.170
|
| [22] |
XIONG X, YU I K M, CHEN S S, et al. Sulfonated biochar as acid catalyst for sugar hydrolysis and dehydration[J]. Catal Today,2018,314:52−61. doi: 10.1016/j.cattod.2018.02.034
|
| [23] |
PARSHETTI G K. , SURYAHARMA M S, PHAM T P T, et al. Heterogeneous catalyst-assisted thermochemical conversion of food waste biomass into 5-hydroxymethylfurfural[J]. Bioresource Technol,2015,178:19−27. doi: 10.1016/j.biortech.2014.10.066
|
| [24] |
YU I K M, TSANG D C W, YIP A C K, et al. Valorization of starchy, cellulosic, and sugary food waste into hydroxymethylfurfural by one-pot catalysis[J]. Chemosphere,2017,184:1099−1107. doi: 10.1016/j.chemosphere.2017.06.095
|
| [25] |
YU I K M, TSANG D C W, YIP A C K, et al. Catalytic valorization of starch-rich food waste into hydroxymethylfurfural (HMF): Controlling relative kinetics for high productivity[J]. Bioresource Technol,2017,237:222−230. doi: 10.1016/j.biortech.2017.01.017
|
| [26] |
YU I K M, TSANG D C W, YIP A C K, et al. Contrasting roles of maleic acid in controlling kinetics and selectivity of Sn(IV)- and Cr(III)-catalyzed hydroxymethylfurfural synthesis[J]. ACS Sustain Chem Eng,2018,6:14264−14274. doi: 10.1021/acssuschemeng.8b02931
|
| [27] |
YU I K M, ONG K L, TSANG D C W, et al. Chemical transformation of food and beverage waste-derived fructose to hydroxymethylfurfural as a value-added product[J]. Catal Today,2018,314:70−77. doi: 10.1016/j.cattod.2018.01.011
|
| [28] |
CAO L, YU I K M, TSANG D C W, et al. Production of 5-hydroxymethylfurfural from starch-rich food waste catalyzed by sulfonated biochar[J]. Bioresource Technol,2018,252:76−82. doi: 10.1016/j.biortech.2017.12.098
|
| [29] |
CAO L, YU I K M, TSANG D C W, et al. Phosphoric acid-activated wood biochar for catalytic conversion of starch-rich food waste into glucose and 5-hydroxymethylfurfural[J]. Bioresource Technol,2018,267:242−248. doi: 10.1016/j.biortech.2018.07.048
|
| [30] |
FLANNELLY T, LOPES M, KUPIANINE L, et al. Non-stoichiometric formation of formic and levulinic acids from the hydrolysis of biomass derived hexose carbohydrates[J]. RSC Adv,2016,6:5797−5804. doi: 10.1039/C5RA25172A
|
| [31] |
PILEDIS F D, TITIRICI M M. Levulinic acid biorefineries: new challenges for efficient utilization of biomass[J]. ChemSusChem,2016,9:562−582. doi: 10.1002/cssc.201501405
|
| [32] |
RITTER S. Biorefinery gets ready to deliver the goods[J]. Chem Eng News,2006,84:34−47.
|
| [33] |
CHEN S S, YU I K M, TSANG D C W, et al. Valorization of cellulosic food waste into levulinic acid catalyzed by heterogeneous Brønsted acids: Temperature and solvent effects[J]. Chem Eng J,2017,327:328−335. doi: 10.1016/j.cej.2017.06.108
|
| [34] |
DUTTA S, YU I K M, FAN J, et al. Critical factors for levulinic acid production from starch-rich food waste: solvent effects, reaction pressure, and phase separation[J]. Green Chem,2022,24:163−175. doi: 10.1039/D1GC01948A
|
| [35] |
XU Z M, LUO J Y, HUANG Y B. Recent advances in the chemical valorization of cellulose and its derivatives into ester compounds[J]. Green Chem,2022,24:3895−3921. doi: 10.1039/D2GC00377E
|
| [36] |
LUIGI B, GEORGIA A, CAMILLA B, et al. Lewis-Brønsted acid catalysed ethanolysis of the organic fraction of municipal solid waste for efficient production of biofuels[J]. Bioresource Technol,2018,266:297−305. doi: 10.1016/j.biortech.2018.06.110
|
| [37] |
DU X L, HE L, ZHAO S, et al. Hydrogen-independent reductive transformation of carbohydrate biomass into γ-valerolactone and pyrrolidone derivatives with supported gold catalysts[J]. Angew Chem Int Ed,2011,123:7961−7965. doi: 10.1002/ange.201100102
|
| [38] |
ISTVAN T H, HASAN M, VIKTORIA F, et al. γ-Valerolactone-a sustainable liquid for energy and carbon-based chemicals[J]. Green Chem,2008,10:238−242. doi: 10.1039/B712863K
|
| [39] |
BOND J Q, ALONSO D M, WANG D, et al. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels[J]. Science,2010,327:1110−1114. doi: 10.1126/science.1184362
|
| [40] |
MOHAMMAD G A, ADAM D, REGINA P. Solvent-free γ-valerolactone hydrogenation to 2-methyltetrahydrofuran catalysed by Ru/C: a reaction network analysis[J]. Green Chem,2014,16:1358−1364. doi: 10.1039/C3GC41803K
|
| [41] |
DU X, BI Q, LIU Y, et al. Tunable copper-catalyzed chemoselective hydrogenolysis of biomass-derived γ-valerolactone into 1, 4-pentanediol or 2-methyltetrahydrofuran[J]. Green Chem,2012,14:935−939. doi: 10.1039/c2gc16599f
|
| [42] |
HUANG Y B, YANG T, LUO Y J, et al. Simple and efficient conversion of cellulose to γ-valerolactone through an integrated alcoholysis/transfer hydrogenation system using Ru and aluminium sulfate catalysts[J]. Catal Sci Technol,2018,8:6252−6262. doi: 10.1039/C8CY01971A
|
| [43] |
CUI J L, TAN J J, DENG T S, et al. Direct conversion of carbohydrates to γ-valerolactone facilitated by a solvent effect[J]. Green Chem,2015,17:3084−3089. doi: 10.1039/C5GC00110B
|
| [44] |
REN H F, ZHU D L, JI F L, et al. One-pot conversion of carbohydrates into gamma-valerolactone under the coordination of heteropoly acid based ionic liquid and Ru/ZrO2 in water media[J]. J Chem Technol Biotechnol,2019,94(7):2355−2363. doi: 10.1002/jctb.6031
|
| [45] |
JIN F M, ENOMOTO H. Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions: chemistry of acid/base-catalysed and oxidation reactions[J]. Energ Environ Sci,2011,4:382−397. doi: 10.1039/C004268D
|
| [46] |
DATTA R, TSAI S P, BONSIGNORE P, et al. Technological and economic potential of poly(lactic acid) and lactic acid derivatives[J]. FEMS Microbiol Rev,1995,16:221−231. doi: 10.1111/j.1574-6976.1995.tb00168.x
|
| [47] |
MARTIN S H, SHUNMUGAVEL S, ESBEN T. Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts[J]. Science,2010,328:602−605. doi: 10.1126/science.1183990
|
| [48] |
YANG X M, LIU Y, LI X X, et al. Synthesis of Sn-containing nanosized beta zeolite as efficient catalyst for transformation of glucose to methyl lactate[J]. Acs Sustain Chem Eng,2018,6:8256−8265. doi: 10.1021/acssuschemeng.8b00177
|
| [49] |
SÁNVHEZ C, SERRANO L, PONTE R L, et al. Bread residues conversion into lactic acid by alkaline hydrothermal treatments[J]. Chem Eng J,2014,250:326−330. doi: 10.1016/j.cej.2014.04.023
|
| [50] |
YANG L, YANG X K, TIAN E, et al. Mechanistic insights into the production of methyl lactate by catalytic conversion of carbohydrates on mesoporous Zr-SBA-15[J]. J Catal,2016,333:207−216. doi: 10.1016/j.jcat.2015.10.013
|




 下载:
下载: