Theoretical study on the catalysis mechanism of sulfur-doped carbon nanotubes in CO2 desorption from monoethanolamine solution
-
摘要: 醇胺法吸收CO2是目前最成熟的碳捕集技术,虽然吸收效率高、稳定性好,但过高的解吸能耗限制其大规模工业推广应用。催化解吸提供了降低CO2解吸能耗的可能性。本工作利用基于密度泛函理论(DFT)的量子化学模拟方法,探索了硫掺杂碳纳米管(S-CNTs)催化单乙醇胺(MEA)溶液吸收-解吸CO2反应机理。通过过渡态搜索发现,以S-CNTs为催化剂的解吸过程,决速步骤的反应能垒降低了1.15 kcal/mol。局部态密度分析表明(PDOS),产物氨基甲酸酯吸附质子化胺MEACOO-_MEAH+和吸收中间产物MEA+COO−中的C、N、O原子在CNTS和S-CNTs表面吸附时PDOS差距较大。此外,与未改性CNTs相比,S-CNTS上电荷密度增加,掺杂的硫原子附近碳原子具有明显的电负性。相比于CNTs,吸收中间产物MEA+COO−和吸收产物MEACOO−-MEAH+均向S-CNTs转移了更多的电荷,表明更多的电荷转移有利于CO2的释放。本工作旨在通过CO2催化解吸机理的研究为催化剂的设计提供一定的理论依据。
-
关键词:
- 掺杂调控 /
- 碳纳米管 /
- 催化MEA解吸CO2机理 /
- DFT计算
Abstract: The CO2 absorption by alkanolamine solution has been applied industrially because of its excellent efficiency. However, the energy consumption for CO2 desorption is high. To reduce the energy consumption, the catalyst is introduced into the alkanolamine capture system. In this study, the catalysis mechanism of sulfur-doped carbon nanotubes (S-CNTs) in CO2 desorption from monoethanolamine (MEA) solution is explored by simulation based on density-functional theory (DFT) calculations. It was found that compared with the single wall carbon nanotubes (CNTs), the adsorption performance of the key substances in the absorption-desorption process on S-CNTs was different, and the adsorption energy of the reactant MEA was reduced by 1.50 kcal/mol, and the adsorption energy of the absorption intermediates MEACOO-_MEAH+ was increased by 2.32 kcal/mol, and the adsorption energy of the absorption product carbamate (MEACOO-)increased significantly. By transition state searching, energy barrier for the rate-determining step was reduced by 1.15 kcal/mol in the desorption process with S-CNTs as the catalyst, suggesting that S-CNTs contributes to amine regeneration. By observing the Mulliken charge of C atoms in the vicinity of S atoms, it was found that the charge of C atoms changed from electroneutral (0.001 eV) to electronegative (−0.325 eV) . The partial density of states (PDOS) of C, N and O atoms from the absorption intermediate MEA+COO− and the absorption product MEACOO−-MEAH+ changes greatly when they adsorbed on CNTs and S-CNTs. In addition, compared with CNTs, the charge density on S-CNTs increases, and the C atoms near the doped S atoms attain obvious electronegativity. Compared with CNTs, the absorption intermediate MEA+COO− and the absorption product MEACOO−-MEAH+ transfer more charges to S-CNTs. This paper is of guiding significance for protecting the environment, maintaining the sustainable development of the energy industry, improving the utilisation rate of raw materials, and reducing the production cost of desorbed CO2. It aims to provide some theoretical basis for the design of catalysts through the study of the catalysis mechanism of S-CNTs in CO2 desorption from monoethanolamine solution. -
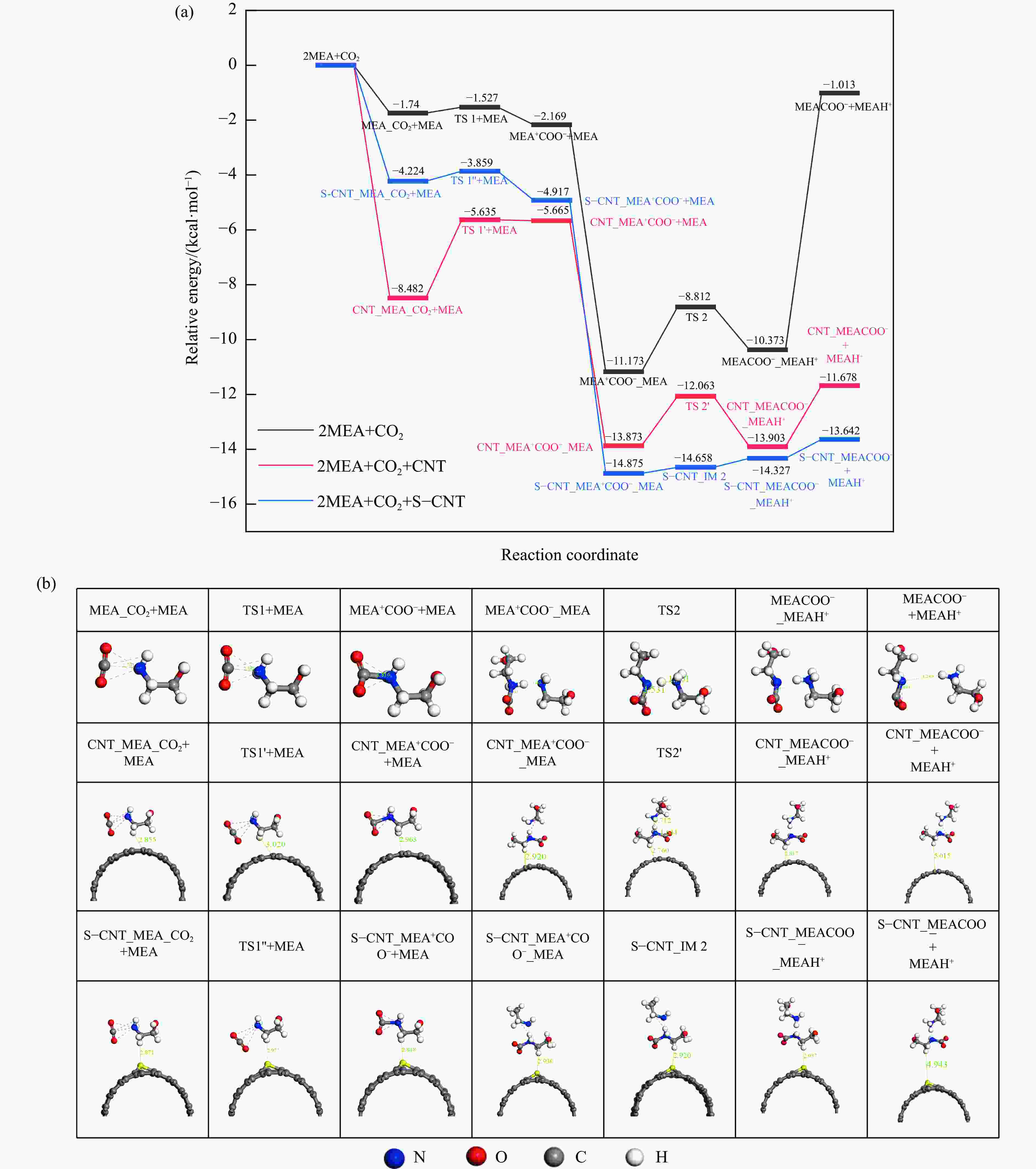
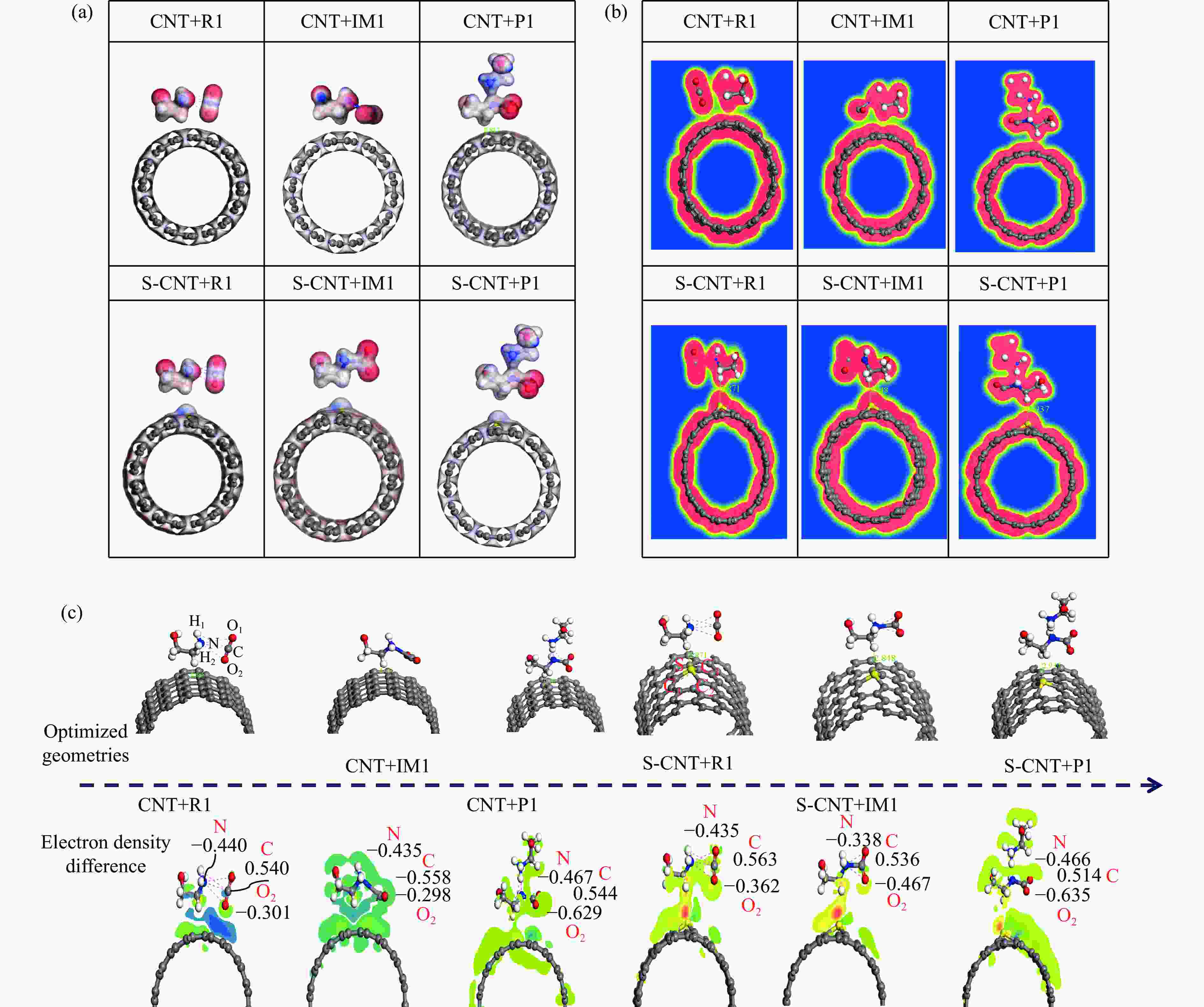
图 3 (a) MEA-CO2-CNTS/S-CNTS催化反应路径结构的静电势图 和(b) 电荷密度图 (c) 差分电荷密度图
Figure 3 (a) Electrostatic potential diagram of the structure of the catalytic reaction pathway of MEA-CO2-CNTS/S-CNTS and (b) Charge density diagram (c) Differential charge density diagramR1 / R refers to the reactant monoethanolamine solution and CO2 in the catalytic desorption reaction, IM 1 refers to the intermediate zwitterion ZW in the catalytic desorption reaction, IM 2 refers to the intermediate ZW and single ethanolamine solution in the catalytic desorption reaction, P1 / P refers to the products carbamate and protonation amine, TS1 and TS2 are transition states.
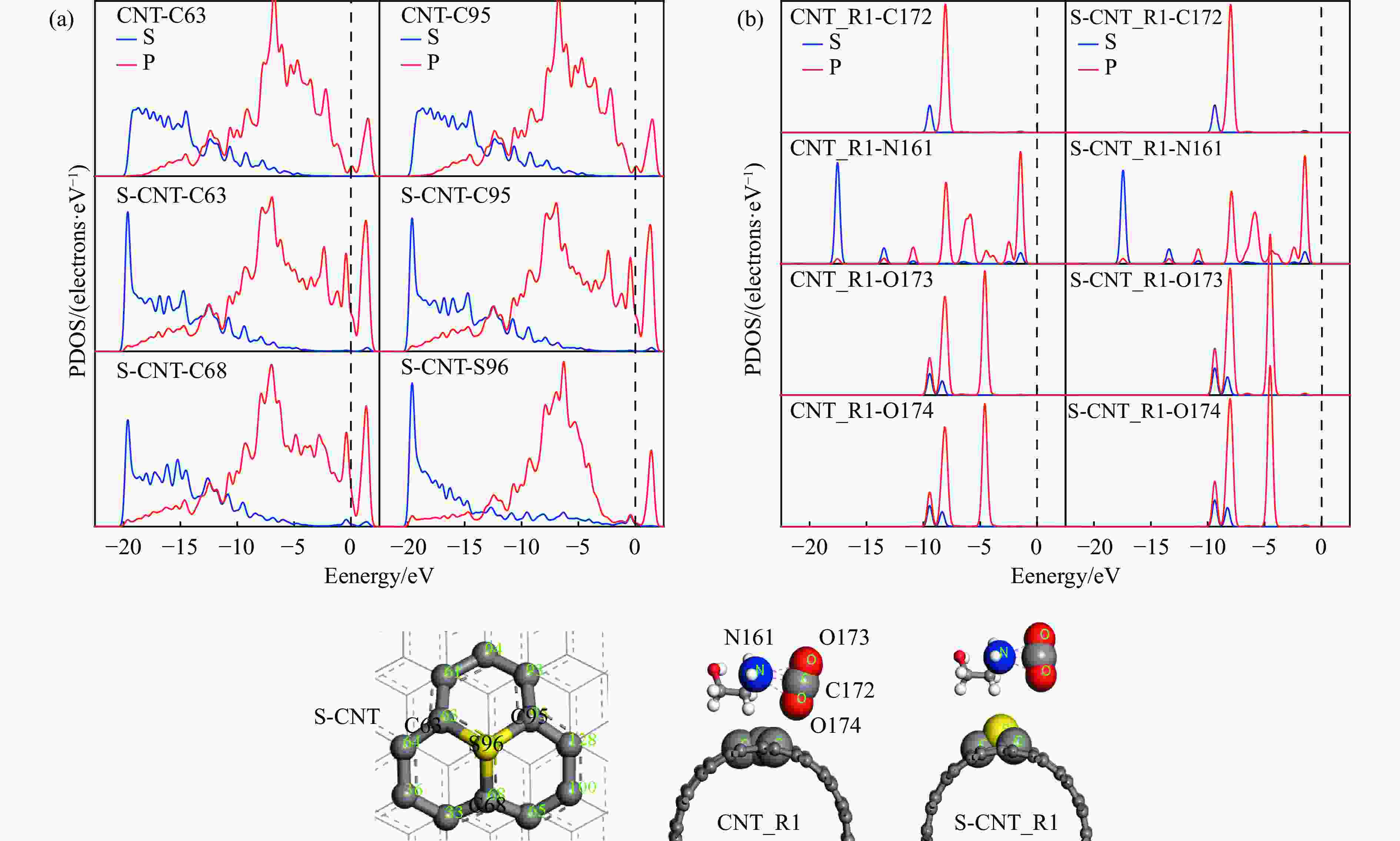
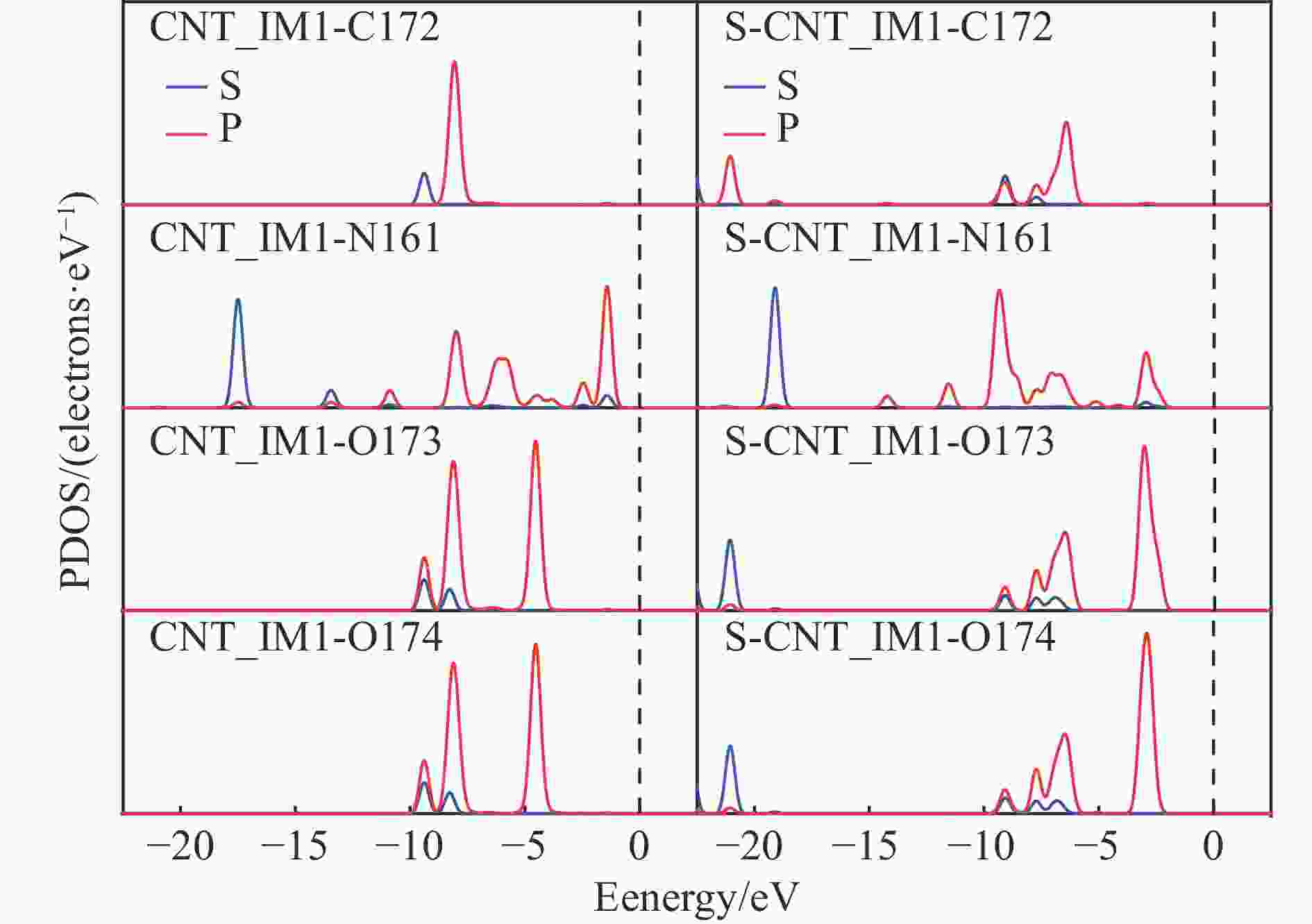
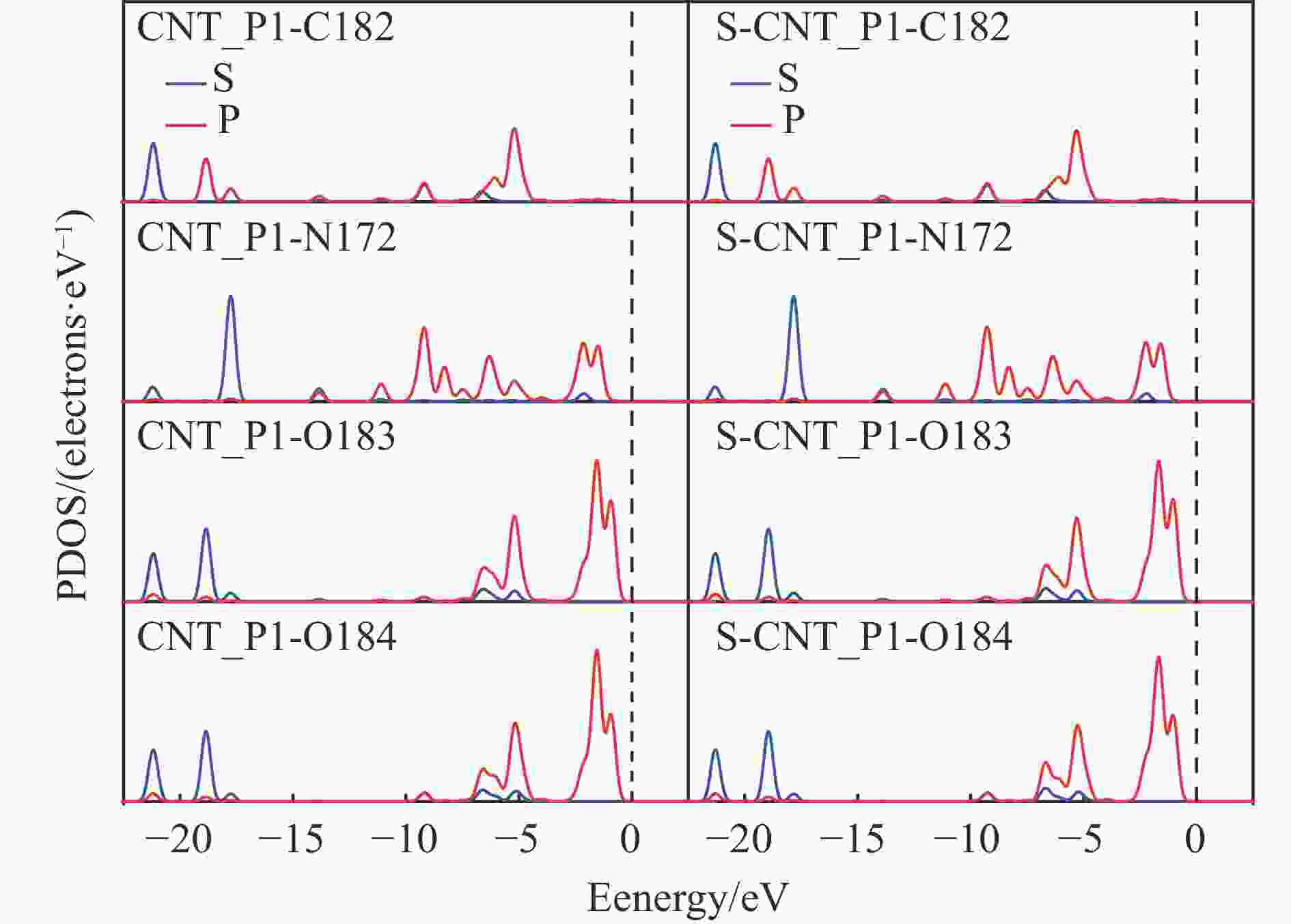
图 4 (a)CNTS/S-CNTS结构中C、S等原子的PDOS图(b)CNTS/S-CNTS_R1催化反应结构中C、N、O等原子的PDOS图
Figure 4 PDOS plots of C, N, O and other atoms in the structure of the CNTS/S-CNTS_R1 catalytic reaction and PDOS maps of C and S and other atoms in the CNTS / S-CNTS structure(The red ball represents oxygen atoms, the gray ball represents C atoms, the yellow ball represents S atoms,the white ball represents H atoms, and the blue ball represents N atoms)
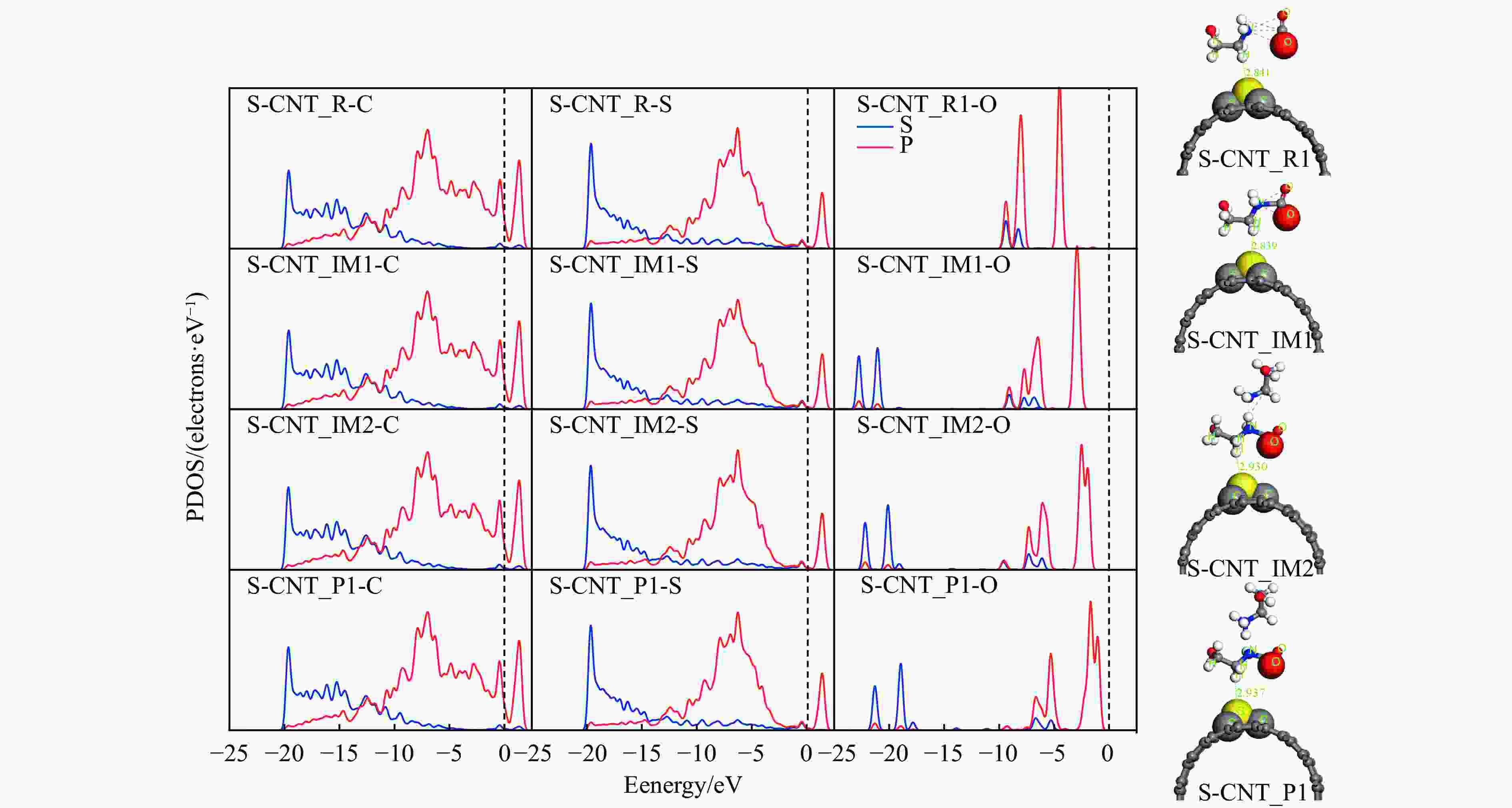
图 7 MEA-CO2-S-CNTS催化反应路径结构中C、S、O原子的PDOS图和 精确分子模型系空间充满式模型(Corey-Pauling-koltun,CPk)模式
Figure 7 The PDOS diagram of C, S and O atoms in structure of MEA-CO2-S-CNTS catalytic reaction pathway and the exact molecular model of S-CNTS adsorption structure are space-filled model (Corey-Pauling-koltun, CPk) mode (Red balls represent oxygen atoms, gray balls represent carbon atoms,yellow balls represent sulfur atoms, and white balls represent hydrogen atoms)
表 1 不同反应物/吸收产物/中间态吸附在碳纳米管/S掺杂碳纳米管上的吸附能
Table 1 Adsorption energy of different reactants/desorption products/intermediate states adsorbed on carbon nanotubes/S-doped carbon nanotubes
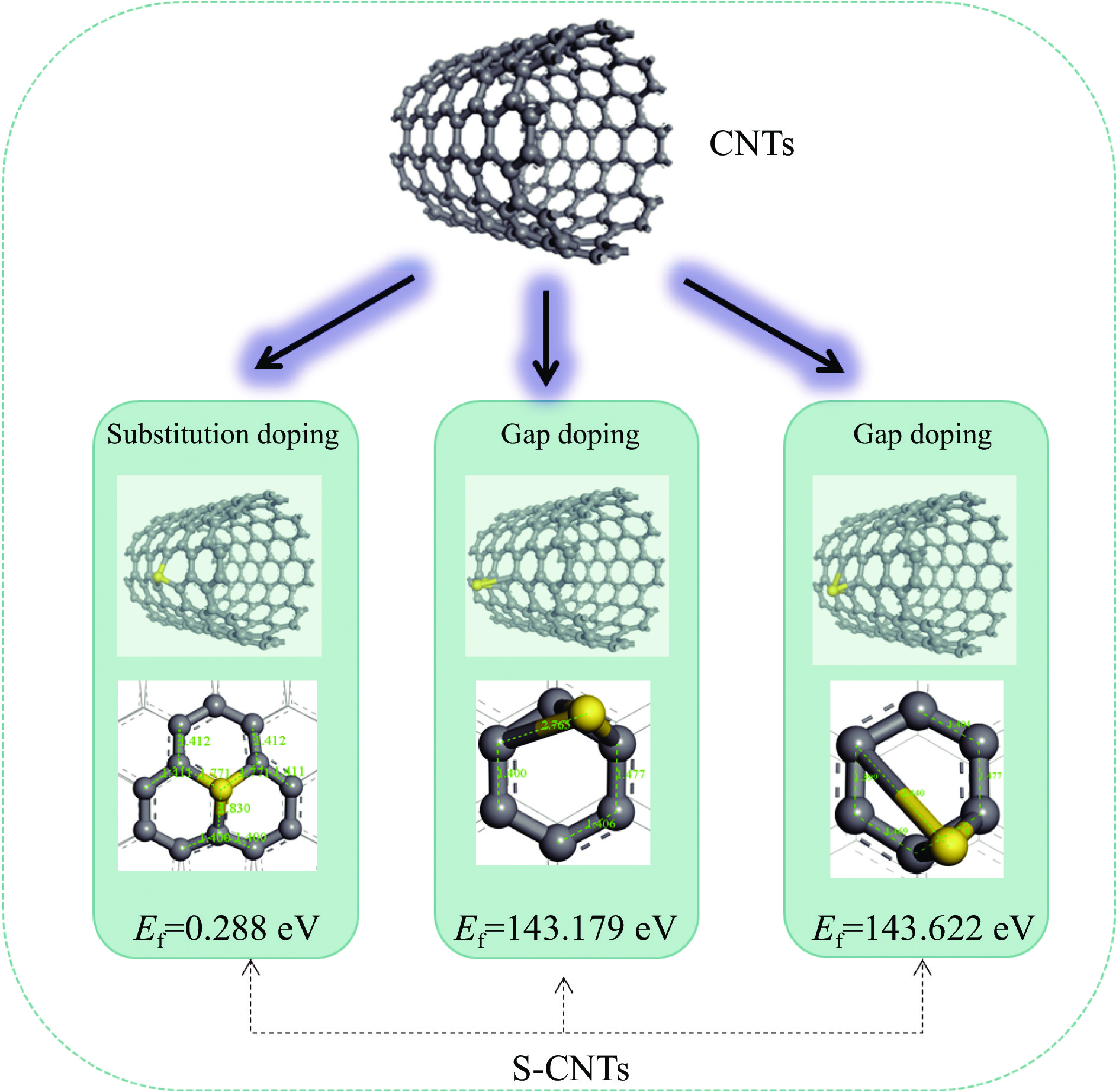
Materials MEA CO2 MEACOO− MEAH+ MEA_CO2 MEA+COO− MEA+COO−_MEA MEACOO−_MEAH+ Ead(CNTs)/(kcal·mol−1) −3.728 −1.489 − −57.228 −5.582 −3.528 −2.700 −3.531 Ead(S-CNTs)/(kcal·mol−1) −2.229 −0.242 −26.322 −56.306 −2.484 −2.748 −2.748 −5.846 注:碳纳米管吸附了吸收产物MEACOO-之后,然后经过结构优化发现MEACOO−分解成了MEA和CO2。 -
[1] ASGHAR U, RAFIQ S, ANWAR A, et al. Review on the progress in emission control technologies for the abatement of CO2, SOx and NOx from fuel combustion[J]. J Environ Chem Eng,2021,9(5):106064. doi: 10.1016/j.jece.2021.106064 [2] WANG T, PARk A-H A, SHI Y, et al. Carbon Dioxide Capture and Utilization—Closing the Carbon Cycle[J]. Energy Fuels,2019,33(3):1693−1693. doi: 10.1021/acs.energyfuels.8b04502 [3] LIANG Z, FU k, IDEM R, et al. Review on current advances, future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents[J]. Chin J Chem Eng,2016,24(2):278−288. doi: 10.1016/j.cjche.2015.06.013 [4] DZIEJARSkI B, SERAFIN J, ANDERSSON k, et al. CO2 Capture Materials: A Review of Current Trends and Future Challenges[J]. Mater Today Sustain, 2023: 100483. [5] WASEEM M, AL-MARZOUQI M, GHASEM N. A review of catalytically enhanced CO2-rich amine solutions regeneration[J]. J. Environ Chem Eng,2023,11(4):110188. doi: 10.1016/j.jece.2023.110188 [6] BHATTI U H, SIVANESAN D, LIM D H, et al. Metal oxide catalyst-aided solvent regeneration: A promising method to economize post-combustion CO2 capture process[J]. J Taiwan Inst Chem. Eng,2018,93:150−157. doi: 10.1016/j.jtice.2018.05.029 [7] SRISANG W, POURYOUSEFI F, OSEI P A, et al. Evaluation of the heat duty of catalyst-aided amine-based post combustion CO2 capture[J]. Chem Eng Sci,2017,170:48−57. doi: 10.1016/j.ces.2017.01.049 [8] ZHANG X, LIU H, LIANG Z, et al. Reducing energy consumption of CO2 desorption in CO2-loaded aqueous amine solution using Al2O3/HZSM-5 bifunctional catalysts[J]. Applied Energy,2018,229:562−576. doi: 10.1016/j.apenergy.2018.07.035 [9] WANG T, YU W, LIU F, et al. Enhanced CO2 Absorption and Desorption by Monoethanolamine (MEA)-Based Nanoparticle Suspensions[J]. Ind Eng Chem. Res,2016,55(28):7830−7838. doi: 10.1021/acs.iecr.6b00358 [10] LAI Q, TOAN S, ASSIRI M A, et al. Catalyst-TiO(OH)2 could drastically reduce the energy consumption of CO2 capture[J]. Nat Commun, 2018, 9(1). [11] LI X, XU Q, LIU Z, et al. Nonacid Carbon Materials as Catalysts for Monoethanolamine Energy-Efficient Regeneration[J]. Environ Sci Technol, 2023: acs. est. 3c01459. [12] YI X, LIU X, FANG J, et al. An atomic/molecular-level strategy for the design of a preferred nitrogen-doped carbon nanotube cathode for Li-O2 batteries[J]. Applied Surface Science,2023,615:156367. doi: 10.1016/j.apsusc.2023.156367 [13] kATTA S S, YADAV S, PRATAP SINGH A, et al. Investigation of pristine and B/N/Pt/Au/Pd doped single-walled carbon nanotube as phosgene gas sensor: A first-principles analysis[J]. Applied Surface Science,2022,588:152989. doi: 10.1016/j.apsusc.2022.152989 [14] ESRAFILI M D, SAEIDI N. Carbon-doped boron nitride nanosheet as a promising catalyst for N2O reduction by CO or SO2 molecule: A comparative DFT study[J]. Applied Surface Science,2018,444:584−589. doi: 10.1016/j.apsusc.2018.03.107 [15] LI X, XUE Q, CHANG X, et al. Effects of Sulfur Doping and Humidity on CO2 Capture by Graphite Split Pore: A Theoretical Study[J]. ACS Appl Mater Interfaces,2017,9(9):8336−8343. doi: 10.1021/acsami.6b14281 [16] SEEMA H, kEMP k C, LE N H, et al. Highly selective CO2 capture by S-doped microporous carbon materials[J]. Carbon,2014,66:320−326. doi: 10.1016/j.carbon.2013.09.006 [17] MESHkAT S S, GHASEMY E, RASHIDI A, et al. Experimental and DFT insights into nitrogen and sulfur co-doped carbon nanotubes for effective desulfurization of liquid phases: Equilibrium & kinetic study[J]. Front Environ Sci. Eng,2021,15(5):109. doi: 10.1007/s11783-021-1397-3 [18] DENIS P A, FACCIO R, MOMBRU A W. Is It Possible to Dope Single-Walled Carbon Nanotubes and Graphene with Sulfur?[J]. ChemPhysChem,2009,10(4):715−722. doi: 10.1002/cphc.200800592 [19] ZHOU J. First-principles study of the effects of Si doping on geometric and electronic structure of closed carbon nanotube[J]. Chinese Science Bulletin,2005,50(17):1823. doi: 10.1360/982005-470 [20] QI C, MA X, NING G, et al. Aqueous slurry of S-doped carbon nanotubes as conductive additive for lithium ion batteries[J]. Carbon,2015,92:245−253. doi: 10.1016/j.carbon.2015.04.028 [21] SEREDYCH M, LÁSZLÓ k, BANDOSZ T J. Sulfur‐Doped Carbon Aerogel as a Metal-Free Oxygen Reduction Catalyst[J]. ChemCatChem,2015,7(18):2924−2931. doi: 10.1002/cctc.201500192 [22] SEREDYCH M, LÁSZLÓ k, RODRÍGUEZ-CASTELLÓN E, et al. S-doped carbon aerogels/GO composites as oxygen reduction catalysts[J]. Journal of Energy Chemistry,2016,25(2):236−245. doi: 10.1016/j.jechem.2016.01.005 [23] DELLEY B. From molecules to solids with the DMol3 approach[J]. J Chem Phys,2000,113(18):7756−7764. doi: 10.1063/1.1316015 [24] CAO N, RAN M F, FENG Y, et al. Density functional theory study of N-doping effect on the stability and activity of Pd/NCNTS catalysts for heck reaction[J]. Applied Surface Science,2020,506:144960. doi: 10.1016/j.apsusc.2019.144960 [25] GRIMME S, ANTONY J, EHRLICH S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. J Chem Phys,2010,132(15):154104. doi: 10.1063/1.3382344 [26] MATSUZAkI Y, YAMADA H, CHOWDHURY F A, et al. Ab Initio Study of CO2 Capture Mechanisms in Aqueous Monoethanolamine: Reaction Pathways for the Direct Interconversion of Carbamate and Bicarbonate[J]. J Phys Chem,2013,117(38):9274−9281. doi: 10.1021/jp406636a [27] GOVIND N, PETERSEN M, FITZGERALD G, et al. A generalized synchronous transit method for transition state location[J]. Comput Mater Sci,2003,28(2):250−258. doi: 10.1016/S0927-0256(03)00111-3 [28] GAO Y, HE X, MAO k, et al. Catalytic CO2 Capture via Ultrasonically Activating Dually Functionalized Carbon Nanotubes[J]. ACS Nano,2023,17(9):8345−8354. doi: 10.1021/acsnano.2c12762 [29] CAO Z, WU X, WEI G, et al. First-Principles Calculations for Adsorption of HF, COF2 , and CS2 on Pt-Doped Single-Walled Carbon Nanotubes[J]. ACS Omega, 2021, 6(37): 23776–23781. [30] REZAIE S, SMEULDERS D M J, LUNA-TRIGUERO A. Enhanced hydrogen storage in gold-doped carbon nanotubes: A first-principles study[J]. Chem Eng J,2023,476:146525. doi: 10.1016/j.cej.2023.146525 -




 下载:
下载: