The study of design and performance improvement of catalysts for the stepwise production of bicyclohexane from benzene and cyclohexene
-
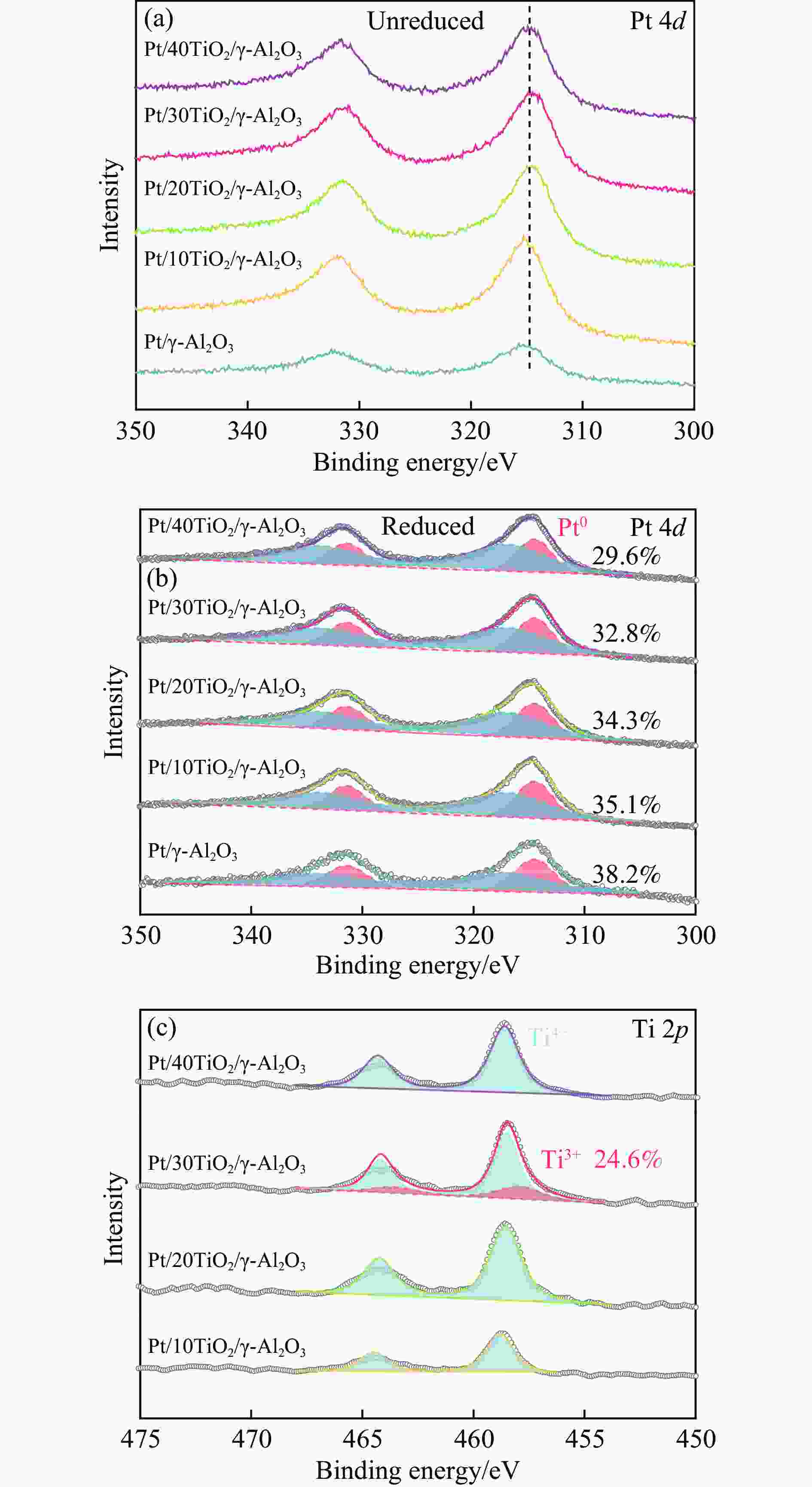
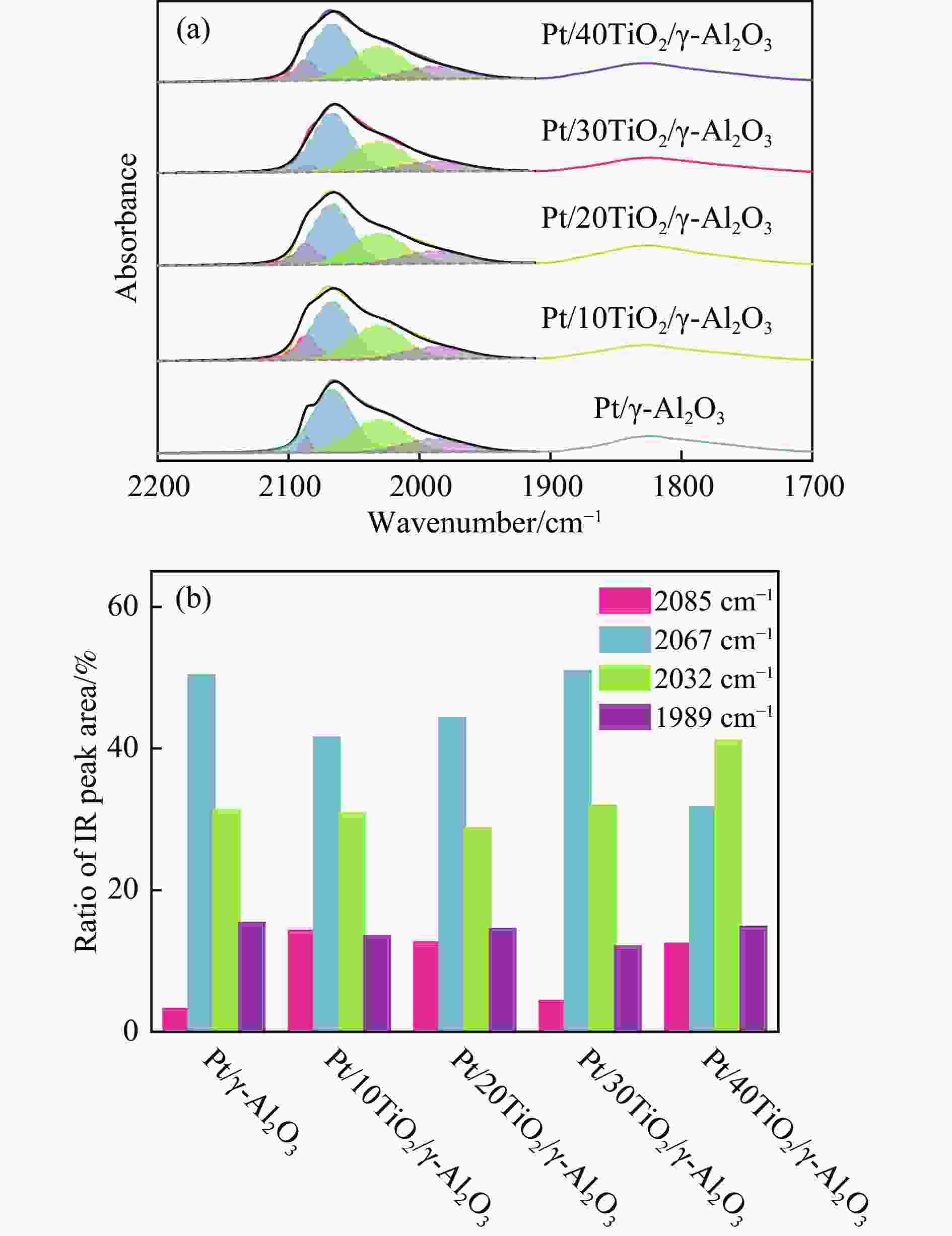
摘要: 联环己烷是一种高储氢密度、低沸点储氢试剂。与联苯加氢相比,苯和环己烯烷基化制环己基苯再加氢是一种有望实现大规模制备联环己烷的途径。在成熟的烷基化技术基础上,需进一步开展高效环己基苯加氢催化剂的研发。本研究首先使用酸化的USY分子筛催化苯和环己烯烷基化至环己基苯,获得100%转化率和产物选择性。进一步通过原子层沉积(ALD)在γ-Al2O3表面预先沉积不同厚度的TiO2膜后再负载铂颗粒制得Pt/TiO2/γ-Al2O3催化剂,研究TiO2膜提升催化剂环己基苯加氢性能机制。TEM、CO脉冲吸附、CO-DRIFTs、准原位XPS、H-D交换和H2-TPR表征显示,与Pt/γ-Al2O3相比,Pt/TiO2/γ-Al2O3催化剂不改变Pt颗粒的分散度,但能够形成新的Pt-TiO2相互作用,提高铂表面电子密度、平面活性位点比例和降低氢溢流能垒,提升环己烷基苯加氢性能。研究为进一步发展联环己烷有机液态储氢试剂提供理论支持。相关金属-载体相互作用调控策略可应用于其他芳香性分子高效加氢催化剂的研制。Abstract: Bicyclohexane is a hydrogen storage reagent with high hydrogen density and low boiling point. Compared with the hydrogenation of biphenyl, the alkylation of benzene and cyclohexene to cyclohexylbenzene and hydrogenation is a promising way to prepare cyclohexane on a large scale. The research and development of high-efficiency cyclohexyl benzene hydrogenation catalyst should be further developed based on mature alkylation technology. This paper used an acidified USY molecular sieve to catalyze the alkylation of benzene and cyclohexene to cyclohexylbenzene, which achieved 100% conversion and selectivity. Furthermore, Pt/TiO2/γ-Al2O3 catalyst is prepared by pre-deposition TiO2 film of different thicknesses on γ-Al2O3 surface and then supported with platinum particles by Atomic layer deposition (ALD). The role of TiO2 film in improving the cyclohexylbenzene hydrogenation performance of the catalyst is studied. TEM、CO pulse chemisorption, CO-DRIFTs, quasi-in situ XPS, H-D exchange, and H2-TPR characterization show that compared with Pt/γ-Al2O3, TiO2 thin films on Pt/TiO2/γ-Al2O3 do not change the dispersion of Pt particles, but can form new Pt-TiO2 interactions. The hydrogenation performance of cyclohexylbenzene was improved by increasing the electron density and the proportion of planar active sites on the surface of platinum and reducing the energy barrier of hydrogen spillover. The research provides theoretical support for further bicyclohexane organic liquid hydrogen storage reagent development. The relevant metal-support interaction regulation strategy can be applied to the development of efficient catalysts for other aromatic molecules hydrogenation.
-
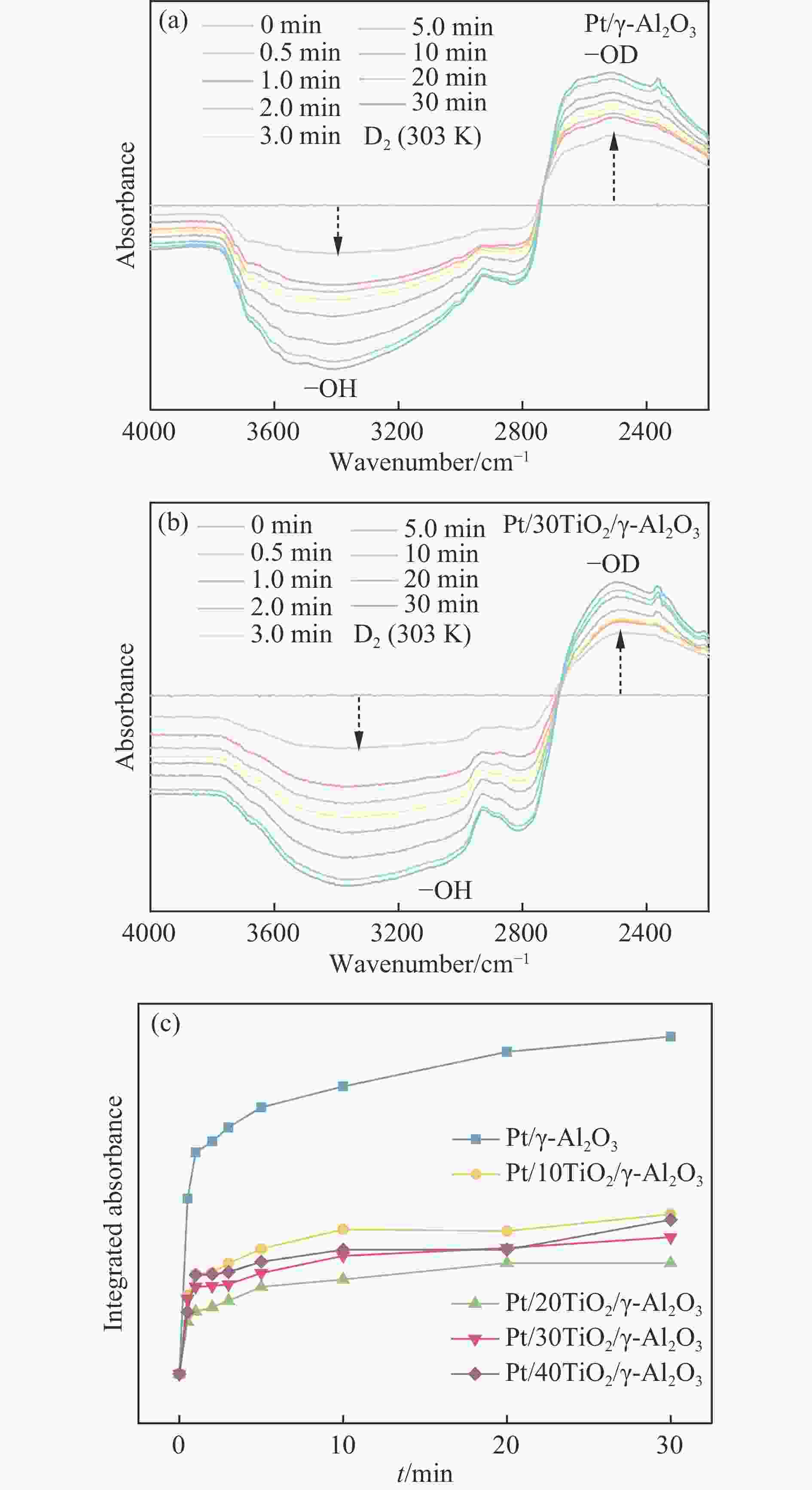
图 5 (a) Pt/γ-Al2O3的H-D交换红外光谱谱图;(b) Pt/30TiO2/γ-Al2O3的H-D交换红外光谱谱图;(c) Pt/γ-Al2O3和Pt/nTiO2/γ-Al2O3催化剂的H-D交换速率对比
Figure 5 (a) FT-IR spectra of the Pt/γ-Al2O3 during H-D exchange, (b) IR spectra of the Pt/30TiO2/γ-Al2O3 during H-D exchange, (c) H-D exchange rates of Pt/γ-Al2O3 and Pt/nTiO2/γ-Al2O3 catalysts
表 1 不同催化剂的苯和环己烯烷基化反应性能
Table 1 Catalytic performance of different catalysts for alkylation of benzene and cyclohexene
Samples Si/Al ratio Conversion/% Selectivity/% cycloxylbenzene 1-methyclopentylbenzene Hβ 5.4 0 — — HY 5 0 — — Dry CT-275 ion-exchange resins — 100 84 16 USY 13−17 0 — — Dry USY 13−17 55.4 100 — Acidified USY 13−17 100 100 — Reaction conditions: 4.6 mmol cyclohexene, 4mL benzene, 150 mg catalyst, 150 ℃ reaction temperature, 3MPa N2, 4 h reaction time. 表 2 不同催化剂Pt和Ti元素负载量、分散度、Pt颗粒尺寸
Table 2 Pt and Ti loading, dispersion and Pt particle diameter of different catalysts
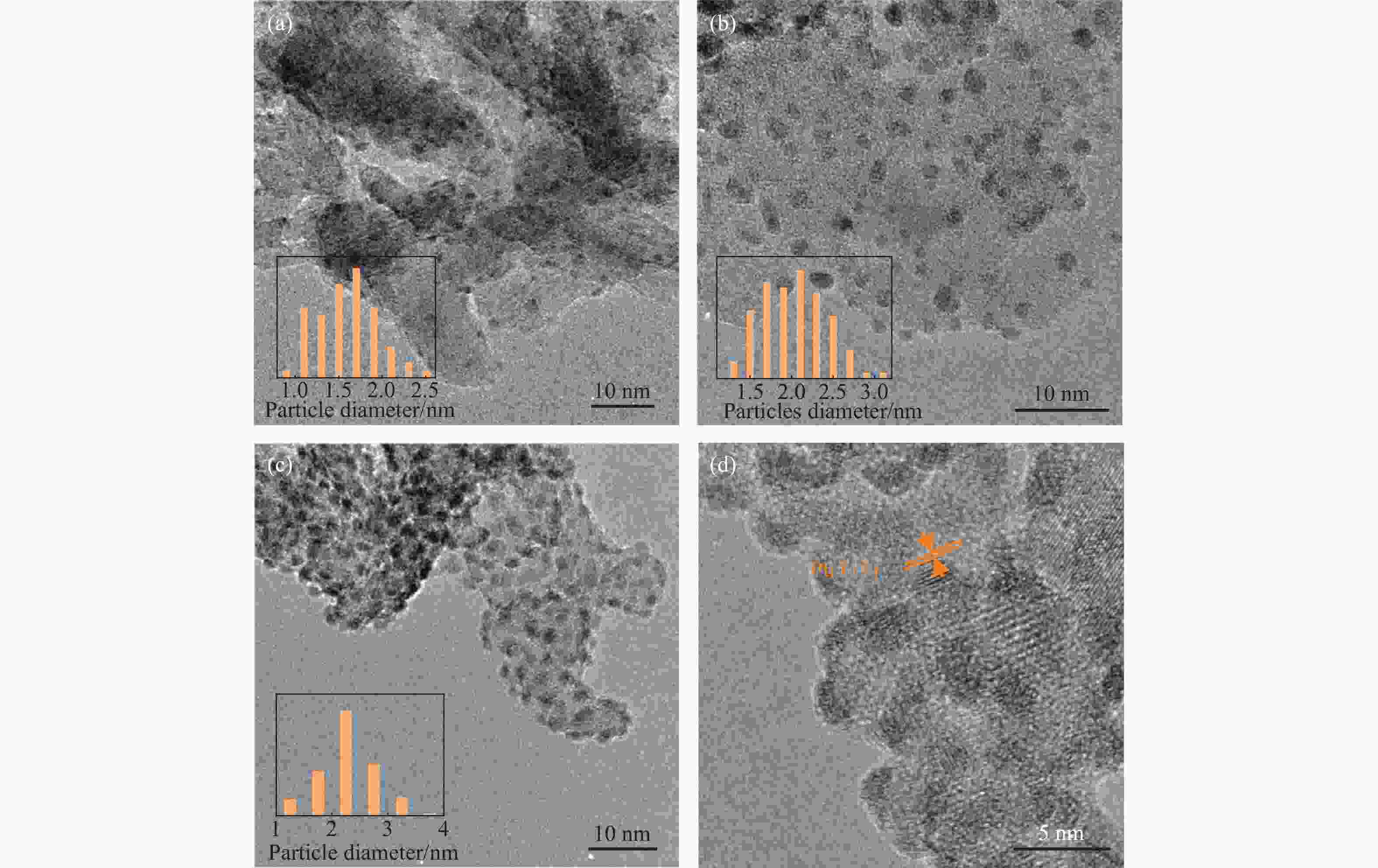
Sample Loading/%a Dispersion/% b dTEM/nm c Pt Ti Pt/γ-Al2O3 9.2 − 19.4 1.6±0.3 Pt/10TiO2/γ-Al2O3 15.4 2.1 19.1 2.0±0.3 Pt/20TiO2/γ-Al2O3 16.0 4.2 15.9 1.9±0.3 Pt/30TiO2/γ-Al2O3 17.8 6.5 16.2 2.3±0.4 Pt/40TiO2/γ-Al2O3 18.3 7.8 15.1 2.0±0.3 Note: a: ICP-OES results; b: CO pulse adsorption; c: TEM particle size distribution statistics. -
[1] BROOM D P, HIRSCHER M. Irreproducibility in hydrogen storage material research[J]. ENERG ENVIRON,2016,9(11):3368−80. doi: 10.1039/C6EE01435F [2] YONGYAN X, YUAN D, WEI L, et al. Research progress of hydrogen energy and metal hydrogen storage materials[J]. SUSTAIN ENERGY TECHN,2022,55:102974. [3] 齐随涛, 黄俊, 陈昊, et al. 有机氢化物可逆储氢循环中脱氢催化剂的研究进展[J]. 化学学报,2012,70(24):2467−74. doi: 10.6023/A12080603QI SuiTao, HUANG Jun, CHEN Hao, et al. Development of dehydrogenation catalyst for reversible hydrogen storage in organic hydrides[J]. ACTA CHIM SINICA,2012,70(24):2467−74. doi: 10.6023/A12080603 [4] DONG Z, MUKHTAR A, LIN H. Heterogeneous catalysis on liquid organic hydrogen carriers[J]. TOP CATAL,2021,64:481−508. doi: 10.1007/s11244-021-01458-5 [5] BINIWALE R B, RAYALU S, DEVOTTA S, et al. Chemical hydrides: A solution to high capacity hydrogen storage and supply[J]. INT J HYDROGEN ENERG,2008,33(1):360−5. doi: 10.1016/j.ijhydene.2007.07.028 [6] CHERNOVA M M, MINAYEV P P, MARTYNENKO Y A, et al. An effect of a support nature and active phase morphology on catalytic properties of Ni-containing catalysts in hydrogenation of biphenyl [J]. RUSS J APPL CHEM+, 2018. [7] 苏文维. Ru/SiO2催化剂的制备及其联苯加氢制联环己烷性能研究 [D], 2021.SU Wenwei. Ru/SiO2 catalysts for the hydrogenation of biphenyl to bicyclohexyl [D]. Zhengzhou university, 2021.) [8] SU W, YANG J, ZHANG M, et al. Highly dispersed and ultra-small Ru nanoparticles deposited on silica support as highly active and stable catalyst for biphenyl hydrogenation[J]. MOL CATA,2021,508:111577. doi: 10.1016/j.mcat.2021.111577 [9] 王闻年, 高焕新, 杨为民. 含钌双功能催化剂上苯加氢烷基化反应性能[J]. 化学反应工程与工艺,2023,39(3):201−10.WANG Wennian, GAO Huanxin, YANG Weimin. Performance of benzene hydroalkylation over Ru-containing bifunctional catalysts[J]. CHEM REACT ENG TECHNOL,2023,39(3):201−10. [10] WANG W, WANG G, GAO H, et al. Progress in synthesis of cyclohexylbenzene and the catalysts[J]. CHEM INDENG PROG,2019,38(1):324−33. [11] FANFEI M, LINHUI D, WEI M, et al. High efficiency catalyst of modified y molecular sieve by rare earth La3+ catalyzed the synthesis of cyclohexylbenzene from benzene and cyclohexene[J]. CATAL LETT,2022,152(3):745−54. doi: 10.1007/s10562-021-03676-8 [12] LU L, RONG Z, DU W, et al. Selective hydrogenation of single benzene ring in biphenyl catalyzed by skeletal Ni[J]. CHEMCATCHEM,2009,1(3):369−71. doi: 10.1002/cctc.200900141 [13] KALENCHUK A N, BOGDAN V I, DUNAEV S F, et al. Dehydrogenation of polycyclic naphthenes on a Pt/C catalyst for hydrogen storage in liquid organic hydrogen carriers[J]. FUEL PROCESS TECHNOL,2018,169:94−100. doi: 10.1016/j.fuproc.2017.09.023 [14] 宋宇淙, 丁晓墅, 赵新强, et al. 保留取代基团的苯环选择加氢反应催化剂研究进展[J]. 精细化工,2023,40(10):2098−111.SONG Yucong, DING Xiaoshu, ZHAO Xinqiang, et al. Research progress on catalysts for selective hydrogenation of benzene rings with tetained substituent groups[J]. FINE CHEMICALS,2023,40(10):2098−111. [15] YU P, YANG Z, GU Z, et al. Catalytic reaction coupling of propane dehydrogenation with nitrobenzene hydrogenation over Pt/Al2O3[J]. CATAL COMMUN,2022,166:106449. doi: 10.1016/j.catcom.2022.106449 [16] CHEN-HUI C, SEUNG YONG L, SANG SOO H. Origin of enhanced toluene hydrogenation by Pt–Ru catalysts for an efficient liquid organic hydrogen carrier[J]. INT J HYDROGEN ENERG,2023,48(86):33590−8. doi: 10.1016/j.ijhydene.2023.05.118 [17] MIN HYE J, JUNGSEOB S, JINHO O, et al. Cerium-modified Pt/Al2O3 for NH3 synthesis by NO reduction with H2[J]. APPL SURF SCI,2023,638:158067. doi: 10.1016/j.apsusc.2023.158067 [18] MENG F, YANG X, ZHAO S, et al. Shifting reaction path for levulinic acid aqueous-phase hydrogenation by Pt-TiO2 metal-support interaction[J]. APPL CATAL B ENVIRON,2023,324:122236. doi: 10.1016/j.apcatb.2022.122236 [19] MENG F, YANG X, ZHAO S, et al. Tailoring the Brønsted acidity of Ti-OH species by regulating Pt-TiO2 interaction[J]. CHEMSUSCHEM,2023,n/a(n/a):e202301410. [20] BERGMAN S L, GRANESTRAND J, TANG Y, et al. In-situ characterization by Near-Ambient Pressure XPS of the catalytically active phase of Pt/Al2O3 during NO and CO oxidation[J]. APPL CATAL B ENVIRON,2017,220:506−11. [21] SHEN M, LV L, WANG J, et al. Study of Pt dispersion on Ce based supports and the influence on the CO oxidation reaction[J]. CHEM ENG J,2014,255:40−8. doi: 10.1016/j.cej.2014.06.058 [22] FANCHUN M, XINCHUN Y, SHICHAO Z, et al. Shifting reaction path for levulinic acid aqueous-phase hydrogenation by Pt-TiO2 metal-support interaction[J]. APPL CATAL B ENVIRON,2022,324:122236. [23] CAROSSO M, FOVANNA T, RICCHEBUONO A, et al. Gas phase vs. liquid phase: monitoring H2 and CO adsorption phenomena on Pt/Al2O3 by IR spectroscopy[J]. CATALSCI TECHNOL,2022,12(4):1359−67. doi: 10.1039/D1CY02233D [24] GE H, ZHANG B, GU X, et al. A Tandem Catalyst with Multiple Metal Oxide Interfaces Produced by Atomic Layer Deposition[J]. ANGEW CHEM INT EDIT,2016,55(25):7081−5. doi: 10.1002/anie.201600799 -




 下载:
下载: