Recent advances in the selective hydrogenation of furfural and its derivatives to pentanediol
-
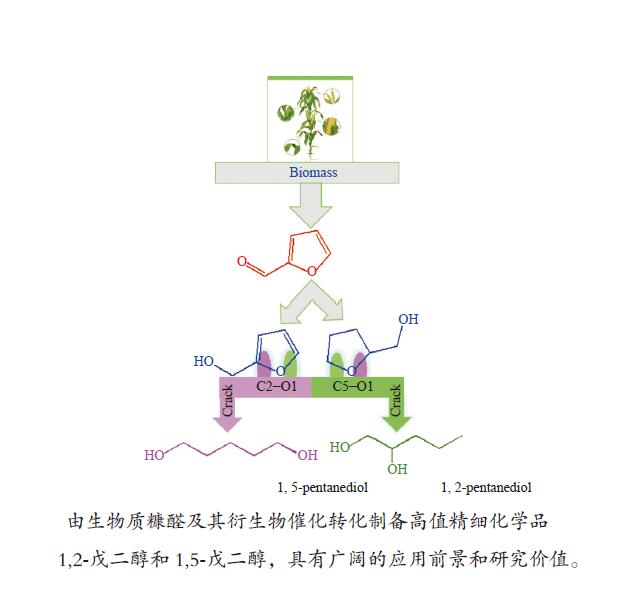
摘要: 1,2-戊二醇(1,2-PeD)和1,5-戊二醇(1,5-PeD)是高附加值精细化学品,用途广泛。以糠醛及其衍生物为原料经催化加氢制备1,2-PeD和1,5-PeD是绿色的生产工艺,具有良好的应用前景和研究价值。本文系统综述了国内外以糠醛及其衍生物糠醇、四氢糠醇为原料制备1,2-PeD和1,5-PeD的研究现状,重点总结了应用于糠醛、糠醇和四氢糠醇催化加氢制备1,2-PeD和1,5-PeD的催化剂,从催化剂类型、不同催化体系辅助酸/碱催化反应机理、活性金属与掺杂过渡金属氧化物间的协同催化、掺杂过渡金属氧化物的酸性以及不同催化体系中催化剂的构效关系等方面进行了详细阐述,并在此基础上对该研究方向的发展趋势进行了展望。为开发新型、高效、稳定催化糠醛及其衍生物加氢催化剂体系提供了理论指导和有益的借鉴。Abstract: 1,2-pentanediol (1,2-PeD) and 1,5-pentanediol (1,5-PeD) are high-value fine chemicals with a wide range of uses. It is a green process with well application prospects and research value for the preparation of 1,2-PeD and 1,5-PeD from furfural and its derivatives. Here, the recent advances of furfural and its derivatives furfuryl alcohol and tetrahydrofurfuryl alcohol in the synthesis of 1,2-PeD and 1,5-PeD were reviewed systematically. We focused on the summary of the catalysts used in the catalytic hydrogenation of furfural, furfuryl alcohol and tetrahydrofurfuryl alcohol to prepare 1,2-PeD and 1,5-PeD. The design and application of the catalysts were elaborated from many aspects, including the catalyst type, the reaction mechanism with assist acid/base in different catalytic systems, the synergistic catalysis between active metals and doped transition metal oxides, the influence of acidity of doped transition metal oxides in the catalyst, the structure-activity relationships and so on. On this basis, the development trend of this research direction is prospected. It provides the theoretical guidance and useful reference for developing a new, efficient and stable catalyst system for the hydrogenation of furfural and its derivatives.
-
Key words:
- furfural /
- furfuryl alcohol /
- tetrahydrofurfuryl alcohol /
- catalytic hydrogenation /
- 1,2-pentanediol /
- 1,5-pentanediol
-
表 1 代表性催化剂催化糠醛(FA)/糠醇(FFA)加氢制备1,2-戊二醇的比较
Table 1 Performance comparison between some representative catalysts of hydrogenolysis FA/FFA to 1,2-PeD
Entry Substrate Catalyst Reaction conditions/
batch reactorConversion
x/ %Selectivity of
1,2-PeD s/%Yield of
1,2-PeD w/%Ref. 1 FA Pt/Al2O3 240 ℃, 2 MPa, 2 h 43.5 33.3 14.5 [17] 2 FA Pt/CeO2 165 ℃, 3 MPa,4 h 100 59.9 59.9 [18] 3 FA Pt/ HT 150 ℃, 3 MPa, 6 h 100 73.0 73.0 [1] 4 FA Rh/OMS-2 160 ℃, 3 MPa,8 h 100 87.0 87.0 [21] 5 FA Pd/MMT-K10 220 ℃, 3.5 MPa,5 h 100 66.0 66.0 [23] 6 FA Ru/Al2O3 200 ℃, 10 MPa, 1 h 100 32 32.0 [24] 7 FFA Ru/MnOx 150 ℃, 1.5 MPa, 4 h 89.2 42.1 37.5 [6] 8 FFA Pt/CeO2 165 ℃, 2 MPa, 24 h 100 77.0 77.0 [25] 9 FFA 10%Cu/Al2O3 140 ℃, 8 MPa, 8 h 85.8 48.1 41.3 [2] 10 FFA 10%Cu-Mg3AlO4.5 140 ℃, 6 MPa, 24 h 100 45.2 45.2 [22] 11 FFA Cu0.8Mg5.2Al2O3 140 ℃, 4 MPa, 8 h 74.1 51 37.8 [26] 表 2 代表性催化剂催化糠醛(FA)/糠醇(FFA)/四氢糠醇(THFA)加氢制备1,5-戊二醇的比较
Table 2 Performance comparison between some representative catalysts of hydrogenolysis FA/FFA/THFA to 1,5-PeD
Entry Substrate Catalyst Reaction conditions Conversion
x/ %Selectivity of
1,5-PeD s/%Yield of 1,5-PeD
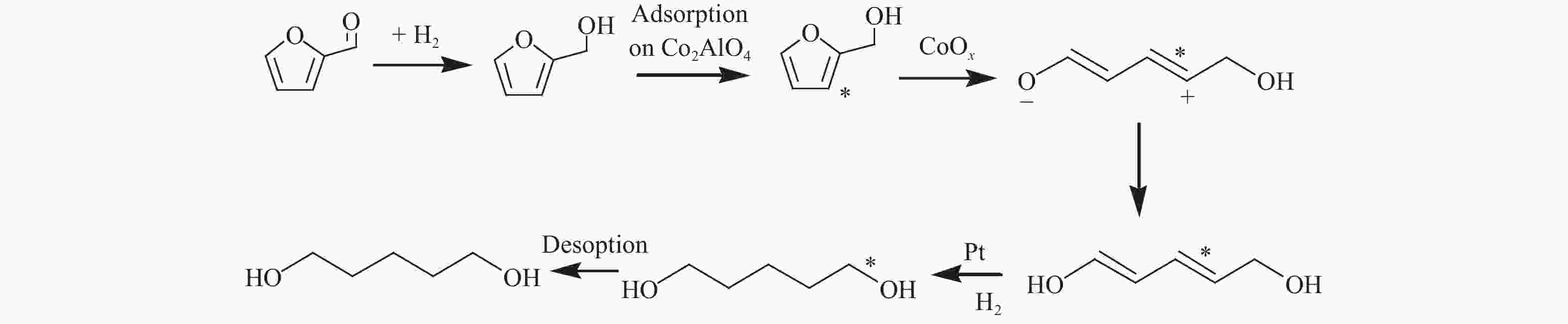
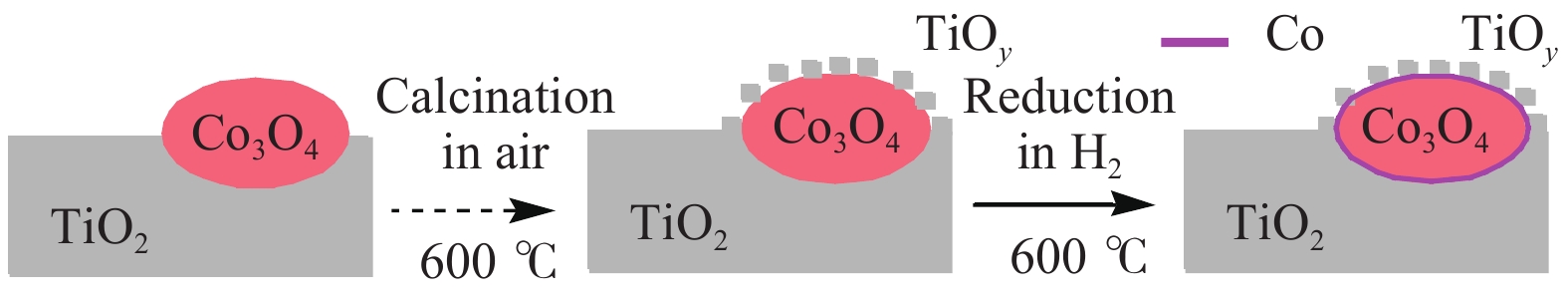
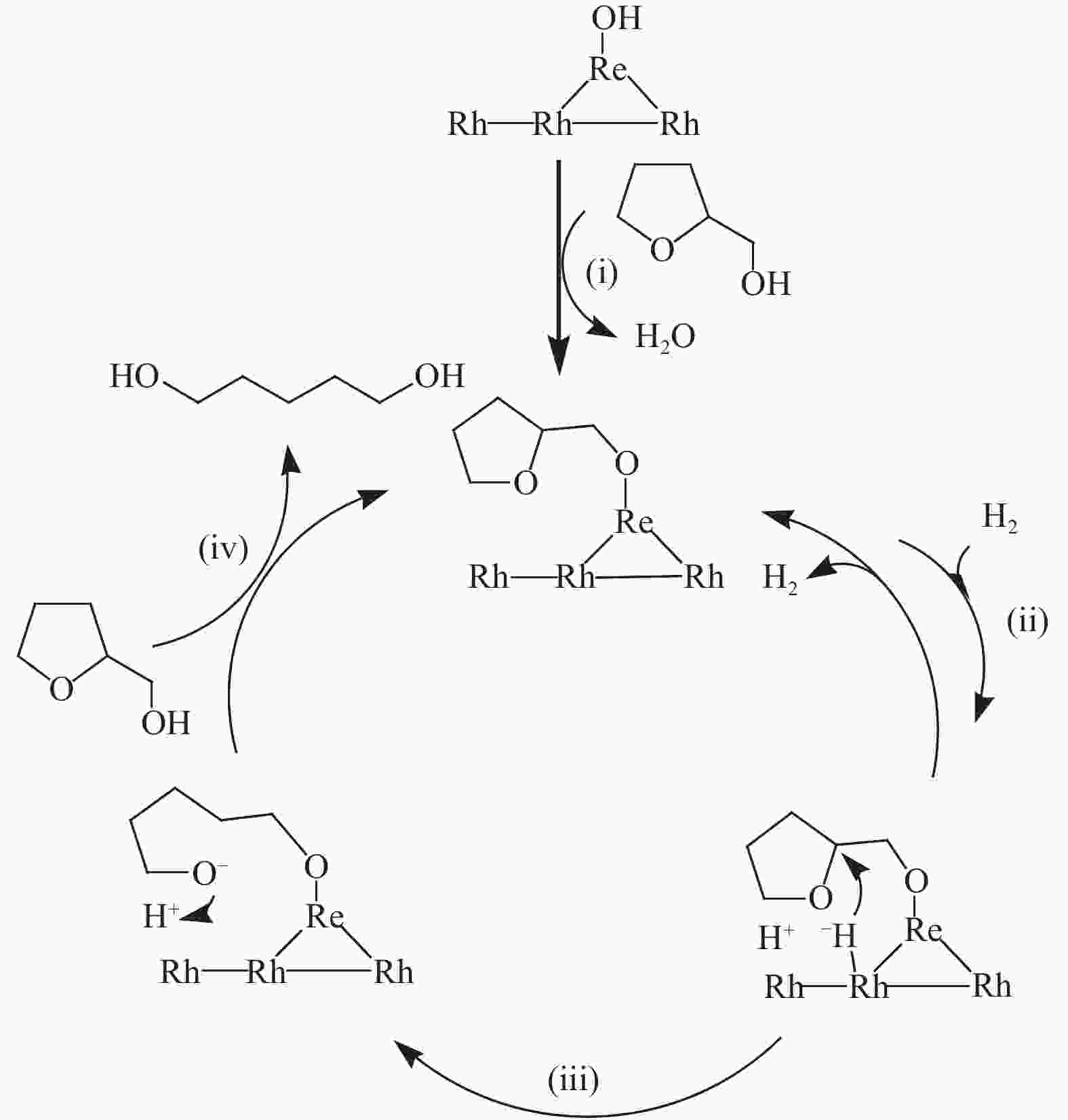
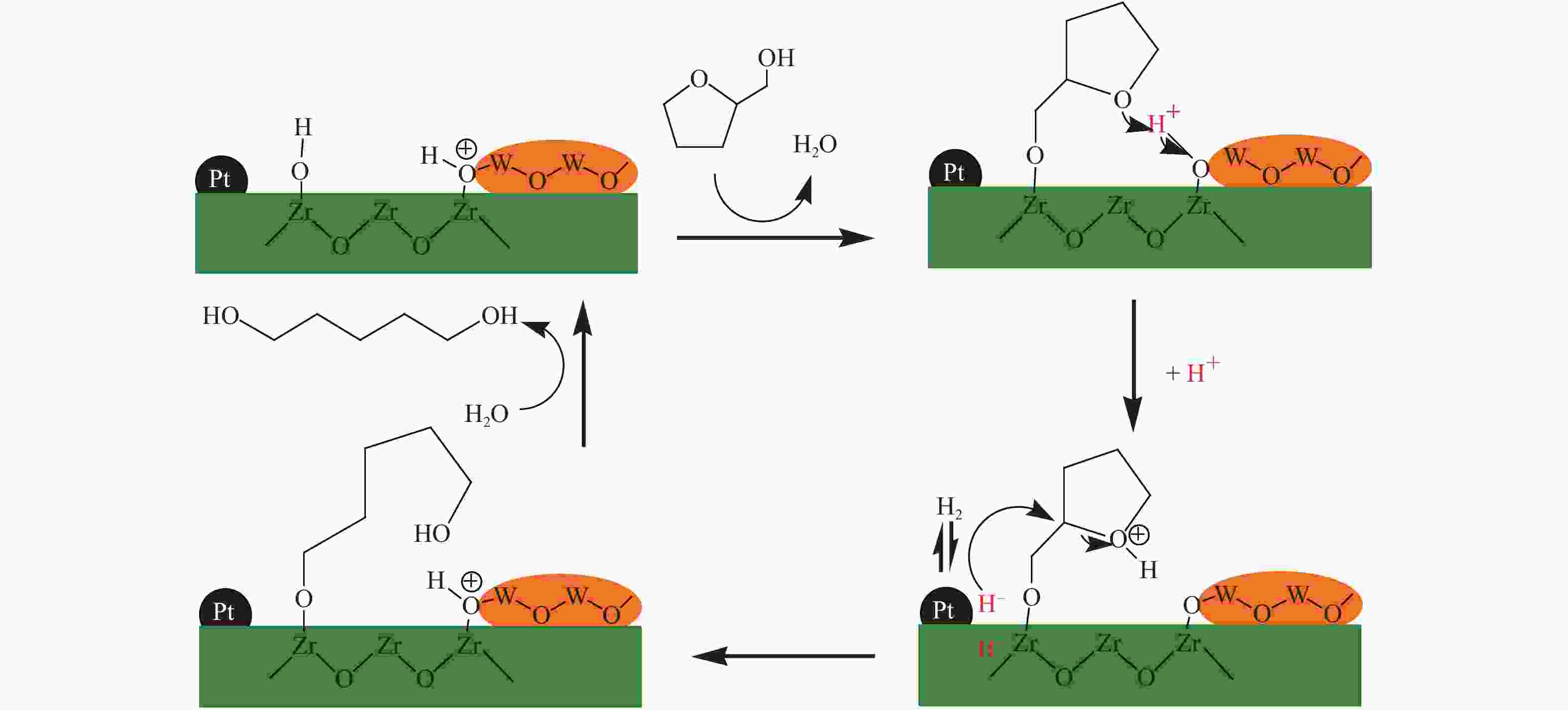
w/%Ref. 1 FA Pt/Co2AlO4 140 ℃, 1.5 MPa, 24 h 100 27.2 27.2 [27] 2 FA Pd-Ir-ReOx/SiO2 40 ℃(8 h),100 ℃(72 h), 8 MPa 100 71.4 71.4 [14] 3 FA Rh-Ir-ReOx/SiO2 40 ℃(8 h),100 ℃ (72 h), 8 MPa 100 78.2 78.2 [13] 4 FFA Co/TiO2 140 ℃, 2.34 MPa,WHSV=5.8 h-1 100 30.3 30.3 [30] 5 FFA Cu-LaCoO3 140 ℃, 6 MPa, 2 h 100 40.3 40.3 [31] 6 FFA 0.1Cu2.9CoAl 160 ℃, 4 MPa, 2 h 98 44.7 43.8 [32] 7 FFA Ni-Y2O3 150 ℃, 2 MPa, 24 h 100 41.9 41.9 [33] 8 FFA Ni-La(OH)3 150 ℃, 2 MPa, 72 h 100 55.8 55.8 [35] 7 THFA Rh-ReOx/SiO2 120 ℃, 8 MPa, 24 h 96 80 76.8 [36] 9 THFA Rh-MoOx/SiO2 100 ℃, 8 MPa, 24 h 94.2 90.3 85.1 [12] 10 THFA Ir-ReOx/SiO2 100 ℃, 8 MPa, 2 h 60.3 94.2 56.8 [38] 11 THFA Rh-ReOx/C 120 ℃, 3.4 MPa, 4 h 47.2 97.2 45.9 [10] 12 THFA Rh-MoOx/C 120 ℃, 3.4 MPa, 4 h 51.6 91.3 47.1 [10] 13 THFA Rh/SiO2 + MoO3 120 ℃, 6 MPa, 20 h 27.9 80.6 22.5 [11] 14 THFA Pt /WO3 @ SiO2 220 ℃, 6 MPa, 24 h 82.9 72.9 60.4 [40] 15 THFA Pt/WO3/ZrO2 150 ℃, 5 MPa, 5 h 56 65 36.4 [42] 16 THFA Pt/Y2O3-WO3-ZrO2 150 ℃, 4 MPa, WHSV=0.2 h-1 88 68 59.8 [43] 17 THFA Ni-WOx/SiO2 250 ℃, 3.4 MPa, 4 h 28.7 47.3 13.6 [44] reaction conditions: entry 1−3, 5−15 and 17 were carried out in batch reactor, entry 4 and entry 16 were carried out in fix-bed reactor -
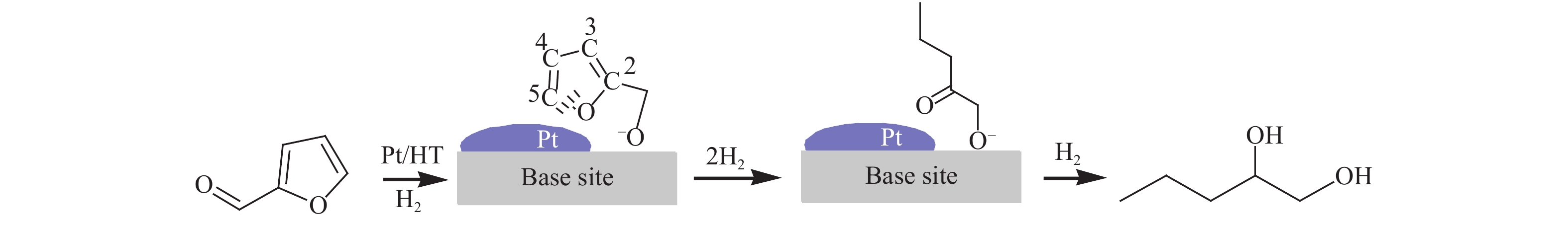
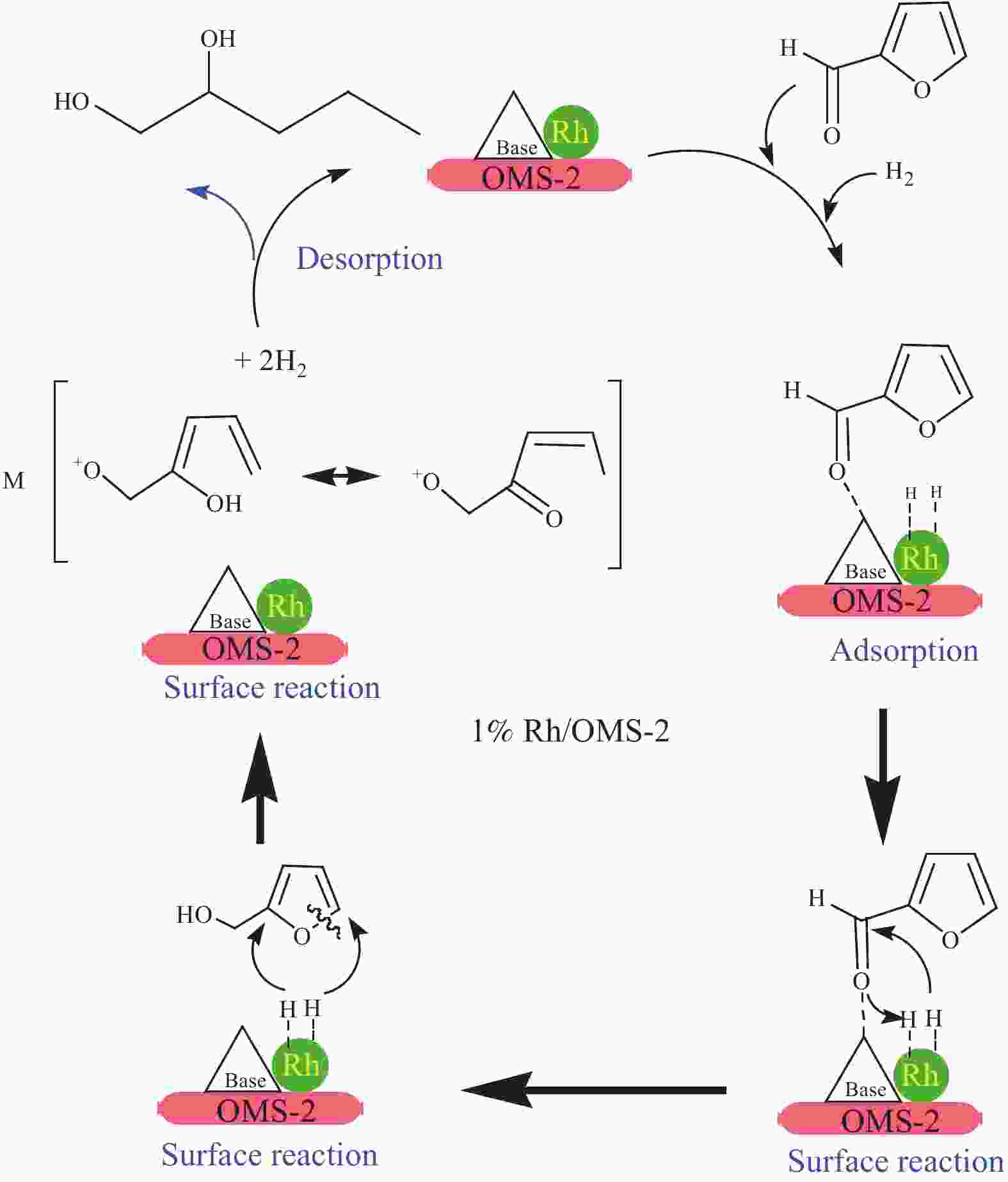
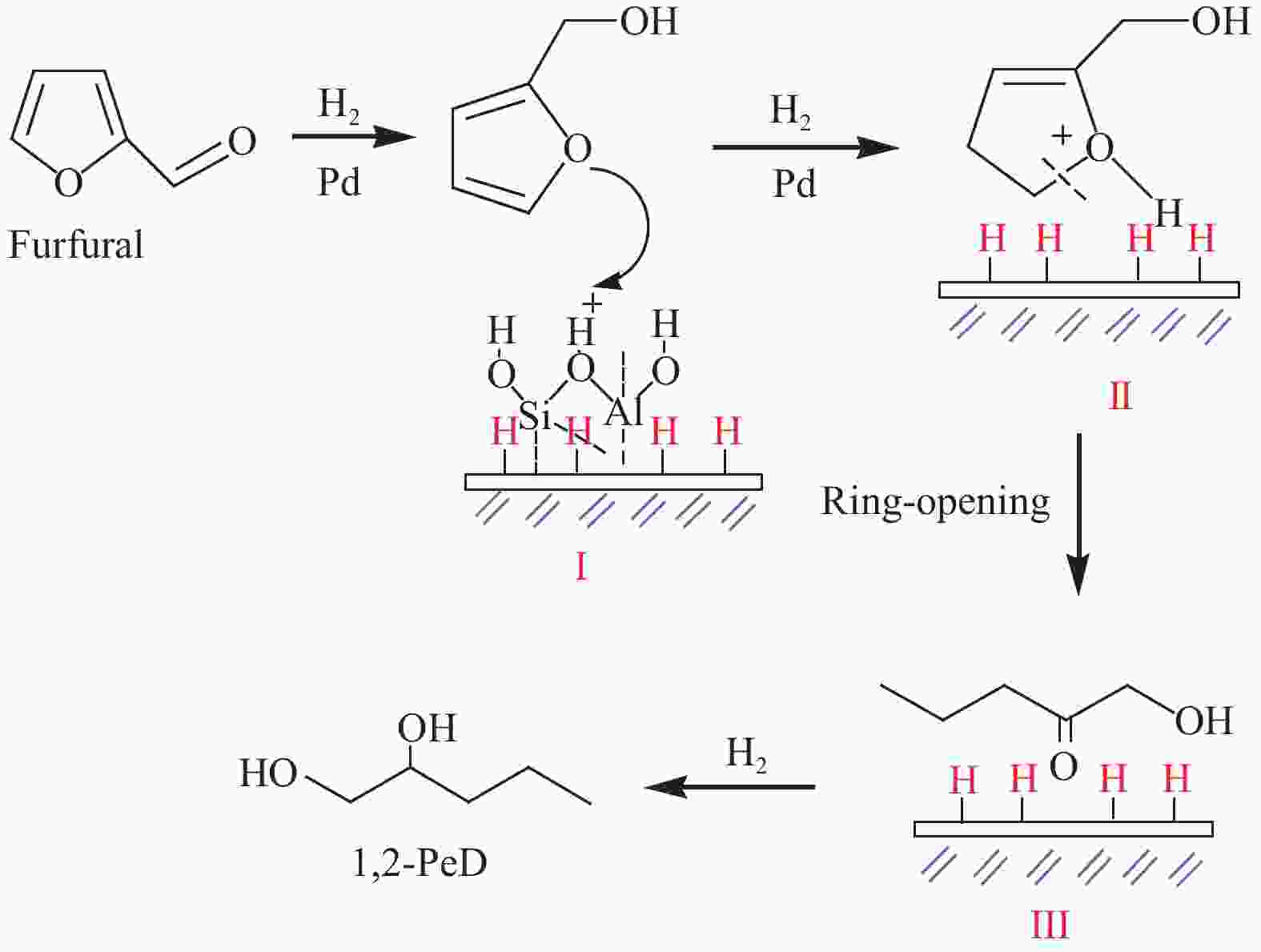
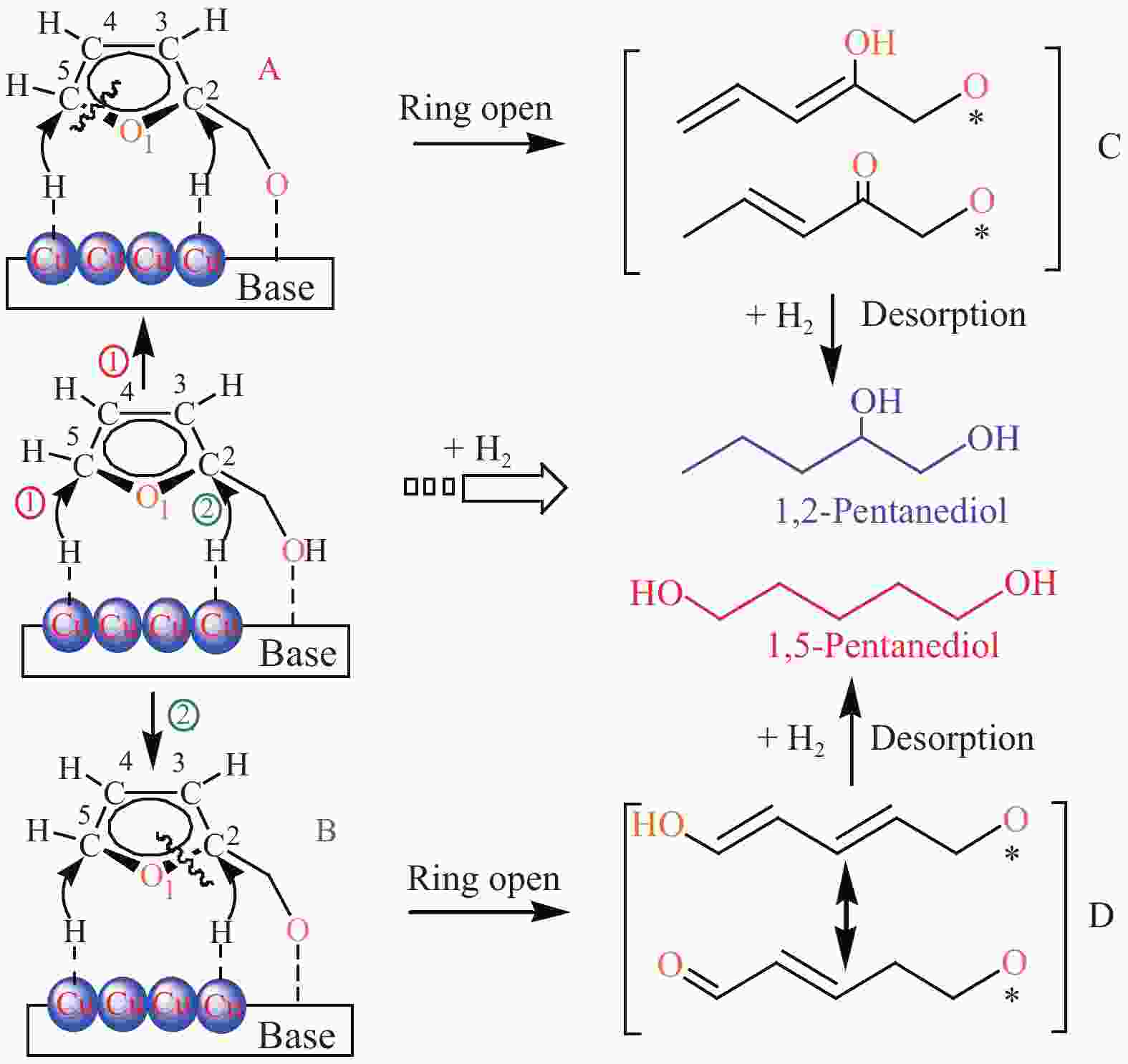
[1] MIZUGAKI T, YAMAKAWA T, NAGATSU Y, MAENO Z, MITSUDOME T, JITSUKAWA K, KANEDA K. Direct transformation of furfural to 1,2-pentanediol using a hydrotalcite-supported platinum nanoparticle catalyst[J]. ACS Sustainable Chem Eng,2014,2(10):2243−2247. doi: 10.1021/sc500325g [2] LIU H, HUANG Z W, KANG H X, XIA C G, CHEN J. Selective hydrogenolysis of biomass-derived furfuryl alcohol into 1,2- and 1,5-pentanediol over highly dispersed Cu-Al2O3 catalysts[J]. Chin J Catal,2016,37(5):700−710. doi: 10.1016/S1872-2067(15)61080-4 [3] SCHLAF M. Selective deoxygenation of sugar polyols to α, ω-diols and other oxygen content reduced materials-a new challenge to homogeneous ionic hydrogenation and hydrogenolysis catalysis[J]. Dalton Trans,2006,39:4645−4653. [4] 李静. 四氢糠醇在 Rh(111)表面上 C−O 键氢解的密度泛函理论研究[D]. 石家庄: 河北科技大学, 2016.LI Jing. Density functional theory study on hydrogenolysis of C−O bond on Rh(111) surface with tetrahydrofurfuryl alcohol[D]. Shijiazhuang: Hebei University of Science and Technology, 2016. [5] BRENTZEL Z J, BARNETT K J, HUANG K, MARAVELIAS P, DUMESIC P, HUBER P. Chemicals from biomass: combining ring-opening tautomerization and hydrogenation reactions to produce 1,5-pentanediol from furfural[J]. ChemSusChem,2017,10(7):1351−1355. [6] ZHANG B, ZHU Y, DING G Q, ZHENG H Y, LI Y W. Selective conversion of furfuryl alcohol to 1,2-pentanediol over a Ru/MnOx catalyst in aqueous phase[J]. Green Chem,2012,14(12):3402−3409. [7] IOELOVICH M. Recent findings and the energetic potential of plant biomass as a renewable source of biofuels - a review[J]. Bioresources,2015,10(1):879−1914. [8] VAMVUKA D. Bio-oil, solid and gaseous biofuels from biomass pyrolysis processes-An overview[J]. Int J Energy Res,2011,35(10):835−862. doi: 10.1002/er.1804 [9] RPEREZ R F, FRAGA M A. Hemicellulose-derived chemicals: one-step production of furfuryl alcohol from xylose[J]. Green Chem,2014,16(8):3942−3950. doi: 10.1039/C4GC00398E [10] CHIA M, PAGÁN-TORRES Y J, HIBBITTS D, HIBBITTS D, TAN Q H, PHAM H N, DATYE A K, NEUROCK M, DAVIS R J, DUMESIC J A. Selective hydrogenolysis of polyols and cyclic ethers over bifunctional surface sites on rhodium-rhenium catalysts[J]. J Am Chem Soc,2011,133(32):12675−12689. doi: 10.1021/ja2038358 [11] GUAN J, PENG G M, CAO Q, MU X D. Role of MoO3 on a rhodium catalyst in the selective hydrogenolysis of biomass-derived tetrahydrofurfuryl alcohol into 1,5-Pentanediol[J]. J Phys Chem C,2014,118(44):25555−25566. doi: 10.1021/jp508313y [12] KOSO S, UEDA N, SHINMI Y, OKUMURA K, KIZUKA T, TOMISHIGE K. Promoting effect of Mo on the hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol over Rh/SiO2[J]. J Catal,2009,267(1):89−92. doi: 10.1016/j.jcat.2009.07.010 [13] LIU S B, AMADA Y, TAMURA M, NAKAGAWA Y, TOMISHIGE K. Performance and characterization of rhenium-modified Rh-Ir alloy catalyst for one-pot conversion of furfural into 1,5-pentanediol[J]. Catal Sci Technol,2014,4(8):2535−2549. doi: 10.1039/C4CY00161C [14] LIU S B, AMADA Y, TAMURA M, NAKAGAWA Y, TOMISHIGE K. One-pot selective conversion of furfural into 1,5-pentanediol over a Pd-added Ir-ReOx/SiO2 bifunctional catalyst[J]. Green Chem,2014,16(2):617−626. doi: 10.1039/C3GC41335G [15] KOCH O, KOECKRITZ A, KANT M, MARTIN A, SCHOENING A, ARMBRUSTER U, BARTOSZEK M, EVERT S, LANGE B, BIENERT R. Method for producing 1, 2-pentanediol: US, 14115706[P]. 2014. [16] KAUFMANN W E, ADAMS R. The use of platinum oxide as a catalyst in the reduction of organic compounds. IV. Reduction of furfural and its derivatives[J]. J Am Chem Soc,1923,45(12):3029−3044. [17] BHOGESWARARAO S, SRINIVAS D. Catalytic conversion of furfural to industrial chemicals over supported Pt and Pd catalysts[J]. J Catal,2015,327:65−77. doi: 10.1016/j.jcat.2015.04.018 [18] TONG T, XIA Q N, LIU X H, WANG Y Q. Direct hydrogenolysis of biomass-derived furans over Pt/CeO2 catalyst with high activity and stability[J]. Catal Commun,2017,101:129−133. doi: 10.1016/j.catcom.2017.08.005 [19] MITSUDOME T, NOUJIMA A, MIKAMI Y, MIZUGAKI T, JITSUKAWA K, KANEDA K. Supported gold and silver nanoparticles for catalytic deoxygenation of epoxides into alkenes[J]. Angew Chem Int Ed,2010,49(32):5545−5548. [20] KAZANSKY V B, BOROVKOV V Y. The unusual properties of small platinum particles supported on basic carriers[J]. Catal Sci Technol,1994,92:275−280. [21] PISAL D S, YADAV G D. Single-step hydrogenolysis of furfural to 1,2-Pentanediol using a bifunctional Rh/OMS2 catalyst[J]. ACS Omega,2019,4(1):1201−1214. [22] LIU H L, HUANG Z W, ZHAO F, CUI F, LI X M, XIA C G, CHEN J. Efficient hydrogenolysis of biomass-derived furfuryl alcohol to 1,2- and 1,5-pentanediols over a non-precious Cu-Mg3AlO4.5 bifunctional catalyst[J]. Catal Sci Technol,2016,6(3):668−671. doi: 10.1039/C5CY01442E [23] DATE N S, CHIKATE R C, ROH H S, RODE C V. Bifunctional role of Pd/MMT-K 10 catalyst in direct transformation of furfural to 1,2-pentanediol[J]. Catal Today,2018,309:195−201. doi: 10.1016/j.cattod.2017.08.002 [24] GÖTZ D, LUCAS M, CLAUS P. C−O bond hydrogenolysis vs. C=C group hydrogenation of furfuryl alcohol: towards sustainable synthesis of 1,2-pentanediol[J]. React Chem Eng,2016,1:161−164. doi: 10.1039/C5RE00026B [25] TONG T, LIU X H, GUO Y, BANIS M N, HU Y F, WANG Y Q. The critical role of CeO2 crystal-plane in controlling Pt chemical states on the hydrogenolysis of furfuryl alcohol to 1,2-pentanediol[J]. J Catal,2018,365:420−428. doi: 10.1016/j.jcat.2018.07.023 [26] 卫彩云, 谭静静, 夏晓丽, 赵永祥. 煅烧温度对CuMgAl催化糠醇加氢制戊二醇的影响[J]. 化工学报,2019,70:1409−1419.WEI Cai-yun, TAN Jing-jing, ZHAO Yong-xiang. Influence of calcination temperature on CuMgAl catalytic performance for hydrogenation of furfuralcohol to pentanediol[J]. CIESC J,2019,70:1409−1419. [27] XU W J, WANG H F, LIU X H, REN J W, WANG Y Q, LU G Z. Direct catalytic conversion of furfural to 1,5-pentanediol by hydrogenolysis of the furan ring under mild conditions over Pt/Co2AlO4 catalyst[J]. Chem Commun,2011,47(13):3924−3926. doi: 10.1039/c0cc05775d [28] MA C Y, MU Z, LI J J, JIN Y G, CHENG J, LU G Q, HAO Z P, QIAO S Z. Mesoporous Co3O4 and Au/Co3O4 catalysts for low-temperature oxidation of trace ethylene[J]. J Am Chem Soc,2010,132(8):2608−2613. doi: 10.1021/ja906274t [29] ADKINS H, CONNOR R J. The catalytic hydrogenation of organic compounds over copper chromite[J]. Am Chem Soc,1931,53(3):1091−1095. doi: 10.1021/ja01354a041 [30] LEE J, BURT S P, CARRERO C A, ALBA-RUBIO A C, RO I, O’NEILL B J, KIM H J, JACKSON D H K, KUECH T F, HERMANS I, DUMESIC J A, HUBER G W. Stabilizing cobalt catalysts for aqueous-phase reactions by strong metal-support interaction[J]. J Catal,2015,330:19−27. doi: 10.1016/j.jcat.2015.07.003 [31] GAO F F, LIU H L, HU X, CHEN J, HUANG Z W, XIA C G. Selective hydrogenolysis of furfuryl alcohol to 1,5- and 1,2-pentanediol over Cu-LaCoO3 catalysts with balanced Cu0-CoO sites[J]. Chin J Catal,2018,39(10):1711−1723. doi: 10.1016/S1872-2067(18)63110-9 [32] SULMONETTI T P, HU B, LEE S, AGRAWAL P K. JONES C W. Reduced Cu-Co-Al mixed metal oxides for the ring-opening of furfuryl alcohol to produce renewable diols[J]. ACS Sustainable Chem Eng,2017,5(10):8959−8969. doi: 10.1021/acssuschemeng.7b01769 [33] WIJAYA H W, KOJIMA T, HARA T, ICHIKUNI N, SHIMAZU P S. Synthesis of 1,5-pentanediol by hydrogenolysis of furfuryl alcohol over Ni-Y2O3 composite catalyst[J]. ChemCatChem,2017,9(14):2869−2874. doi: 10.1002/cctc.201700066 [34] YAO S X, WANG X C, JIANG Y J, WU F, CHEN X G, MU X D. One-step conversion of biomass-derived 5-hydroxymethylfurfural to 1,2, 6-hexanetriol over Ni-Co-Al mixed oxide catalysts under mild conditions[J]. ACS Sustainable Chem Eng,2014,2(2):173−180. [35] WIJAYA H W, SATO T, TANGE H, HARA T, ICHIKUNI N, SHIMAZU S P. Hydrogenolysis of furfural into 1,5-pentanediol by employing Ni-M (M = Y or La) composite catalysts[J]. Chem Lett,2017,46(5):744−746. doi: 10.1246/cl.170129 [36] KOSO S, FURIKADO I, SHIMAO A, MIYAZAWA T, KUNIMORI K, TOMISHIGE K. Chemoselective hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol[J]. Chem Comm,2009,106:2035−2037. [37] KOSO S, NAKAGAWA Y, TOMISHIGE K. Mechanism of the hydrogenolysis of ethers over silica-supported rhodium catalyst modified with rhenium oxide[J]. J Catal,2011,280(2):221−229. doi: 10.1016/j.jcat.2011.03.018 [38] CHEN K Y, MORI K, WATANABE H, NAKAGAWA Y, TOMISHIGE K. C−O bond hydrogenolysis of cyclic ethers with OH groups over rhenium-modified supported iridium catalysts[J]. J Catal,2012,294:171−183. doi: 10.1016/j.jcat.2012.07.015 [39] TOMISHIGE K, NAKAGAWA Y, TAMURA M. Selective hydrogenolysis and hydrogenation using metal catalysts directly modified with metal oxide species[J]. Green Chem,2017,19(13):2876−2924. doi: 10.1039/C7GC00620A [40] KUANG B F, ZHANG Q, FANG Y X, BAI Y, QIU S B, WU P, QIN Y L, WANG T J. Ring opening of cyclic ether for selective synthesis of renewable 1,5-pentanediol over Pt/WO3@SiO2 catalysts[J]. Ind Eng Chem Res,2020,59(20):9372−9381. [41] WANG C, LEE J D, JI Y C, ONN T M, LUO J, MURRAY C B, GORTE R J. A study of tetrahydrofurfuryl alcohol to 1,5-pentanediol over Pt-WOx/C[J]. Catal Lett,2018,148:1047−1054. doi: 10.1007/s10562-018-2323-6 [42] FENG S X, NAGAO A, AIHARA T, MIURA H, SHISHIDO T. Selective hydrogenolysis of tetrahydrofurfuryl alcohol on Pt/WO3/ZrO2 catalysts: Effect of WO3 loading amount on activity[J]. Catal Today,2018,303:207−212. doi: 10.1016/j.cattod.2017.08.058 [43] 杨晓, 陈长林. Y2O3对 Pt /WO3 -ZrO2催化剂催化四氢糠醇加氢制备 1,5-戊二醇的影响[J]. 南京工业大学学报,2019,41(2):149−154.YANG Xiao, CHEN Chang-lin. The effect of Y2O3 on Pt /WO3 -ZrO2 catalyst for the hydrogenation of tetrahydrofurfuryl alcohol to 1,5-pentanediol[J]. J Nanjing Technol Univ,2019,41(2):149−154. [44] SOGHRATI E, POH C K, DU Y H, GAO F, KAWI P S, BORGNA A. C-O hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-Pentanediol Over bi-functional nickel-tungsten catalysts[J]. ChemCatChem,2018,10(20):4652−4664. doi: 10.1002/cctc.201800783 -




 下载:
下载: