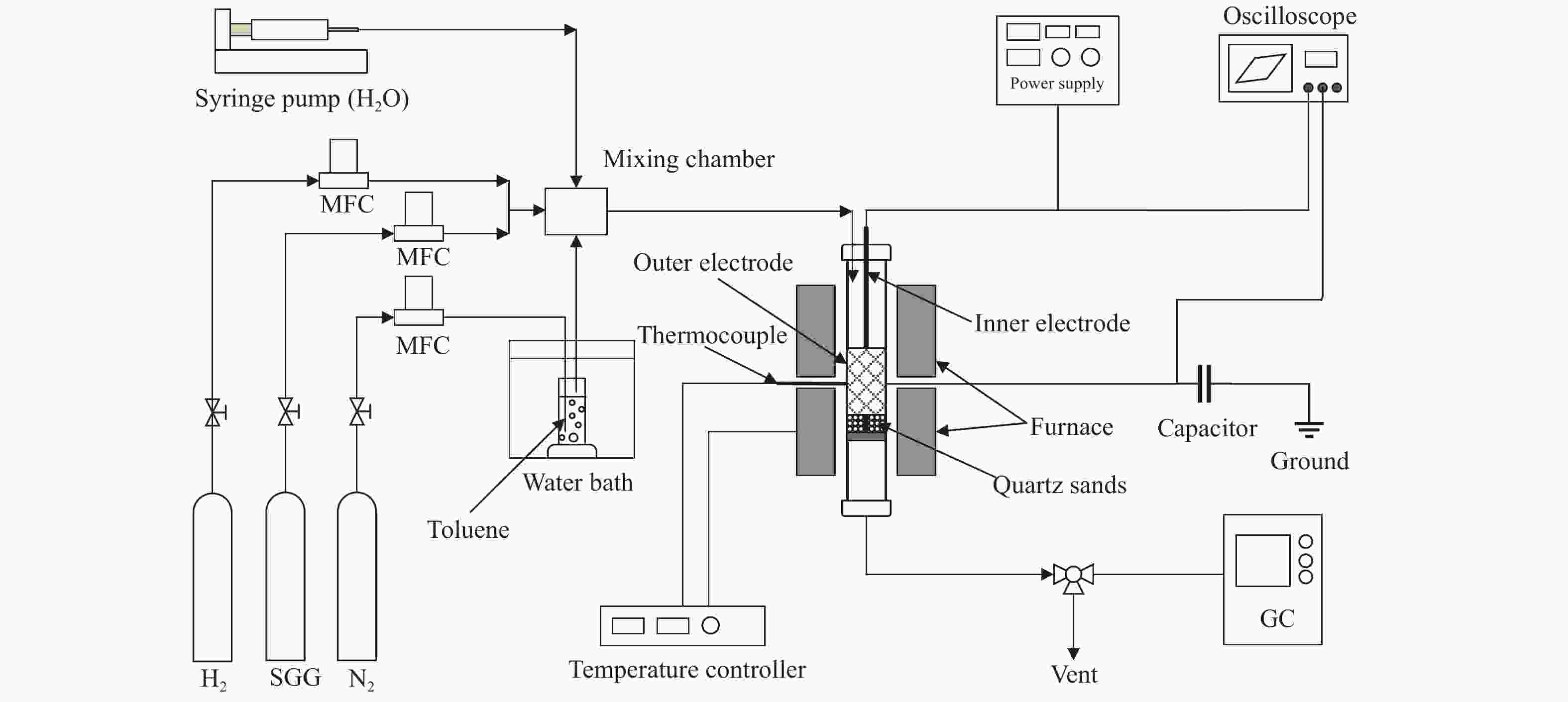
Performance study on simultaneous tar removal and bio-syngas methanation by combining catalysis with nonthermal plasma
-
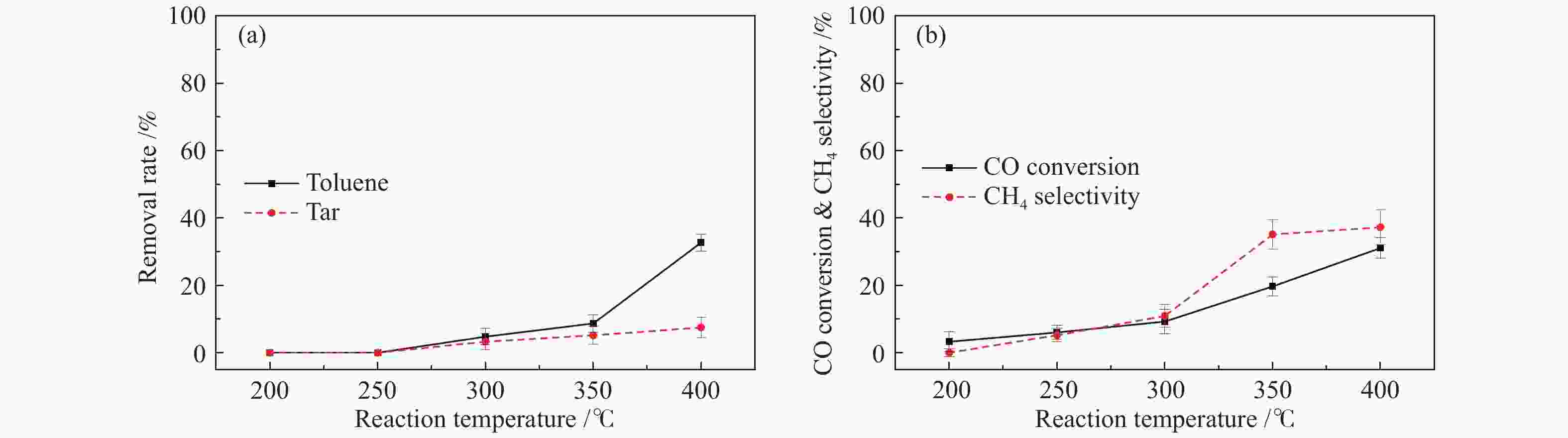
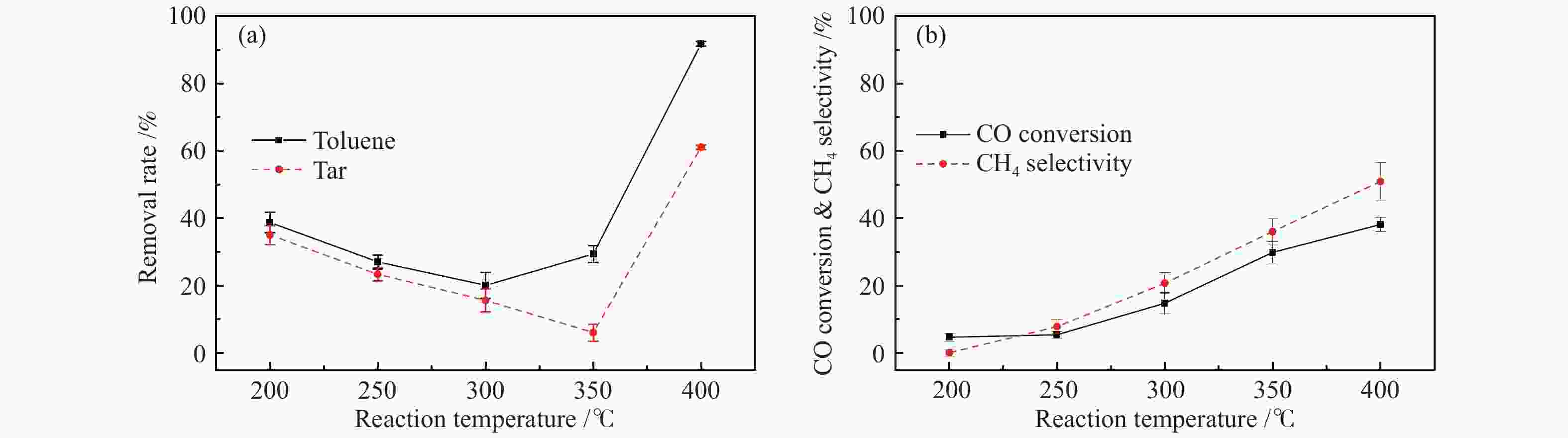
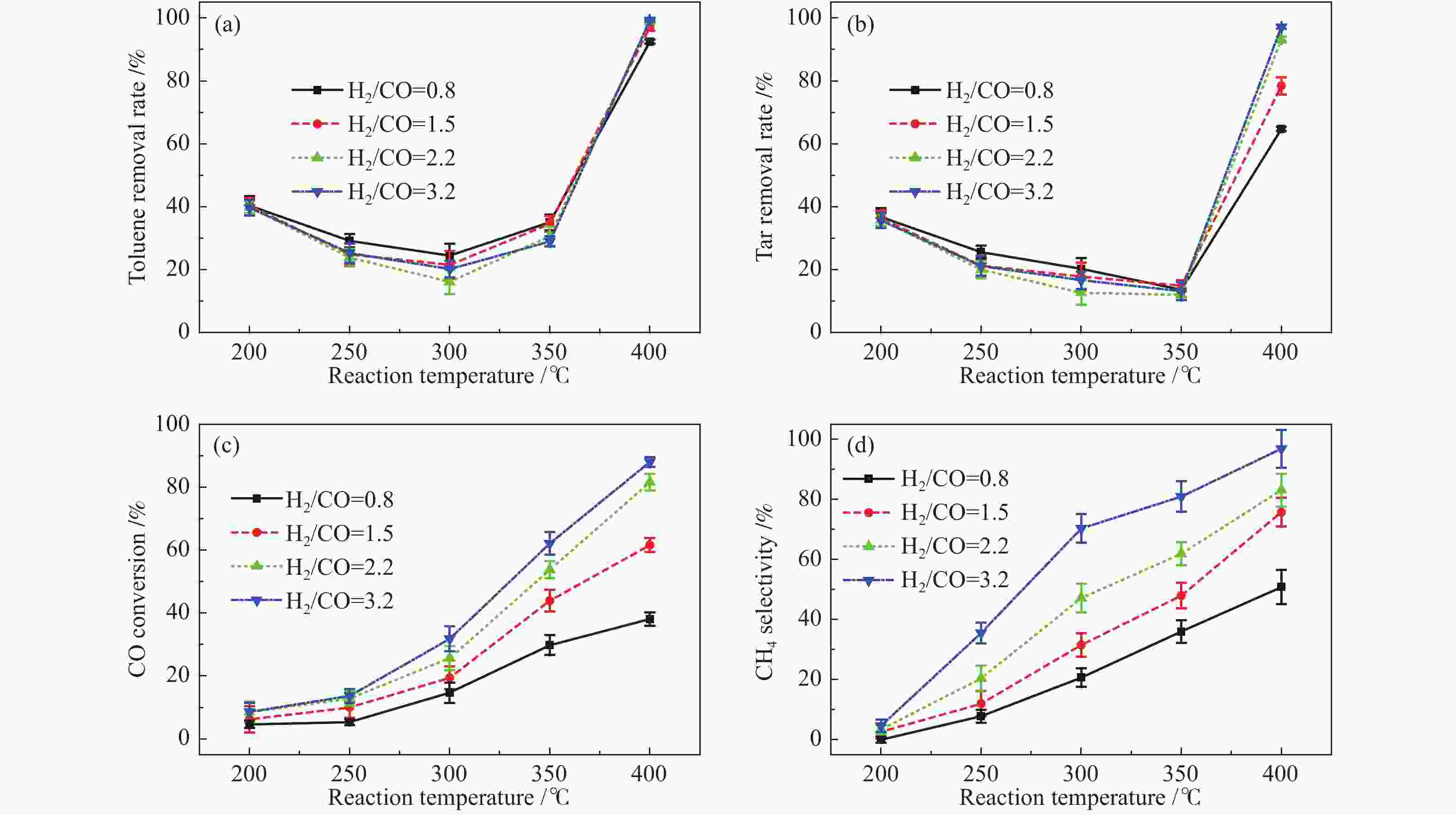
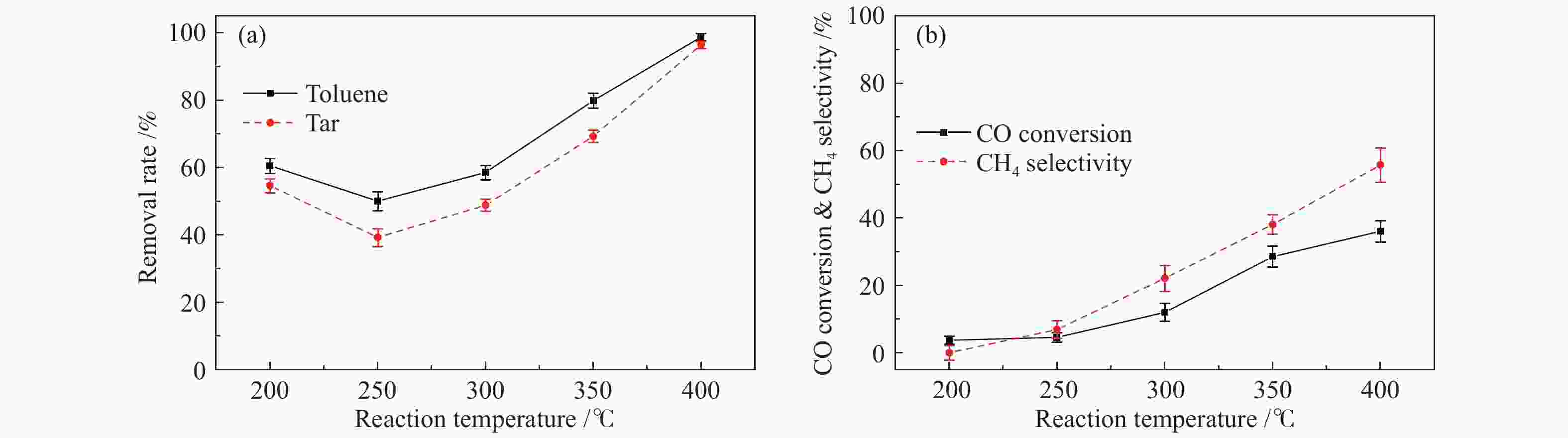
摘要: 以含甲苯的模拟气化燃气(SGG)为对象,在介质阻挡放电耦合Ni/γ-Al2O3反应器上开展同时甲苯脱除及SGG甲烷化实验研究。考察了反应温度、H2/CO比、H2O添加的影响。结果表明,等离子体耦合催化可在400 ℃实现高效的同时甲苯脱除与SGG甲烷化。H2/CO比为3.2时,甲苯脱除率与焦油脱除率可达100%和97%,CO转化率与CH4选择性可达88%和97%,甲苯脱除与SGG甲烷化过程能量效率可达9.7 g/(kW·h)和17.3 mol/(kW·h)。高H2/CO比与H2O添加可促进甲苯脱除和SGG甲烷化,降低催化剂积炭量并提升积炭石墨化程度,其中,高H2/CO比还可提升SGG热值,获得高甲苯脱除及SGG甲烷化过程能量效率;而H2O添加会降低热值且难获得高CH4选择性,同时不利于SGG甲烷化过程能量效率的提升。此外,SGG甲烷化会抑制甲苯的脱除,而甲苯因浓度较低对甲烷化过程的影响较小。Abstract: In this work, simultaneous toluene removal and syngas methanation using the combination of packed-bed dielectric barrier discharge and Ni/γ-Al2O3 catalyst were conducted with the research object of simulated gasification gas (SGG) containing toluene. The effects of reaction temperature, H2/CO ratio and H2O addition on the reaction performances of both toluene removal and SGG methanation were investigated. The results show that high-efficiency simultaneous toluene removal and SGG methanation can be achieved at 400°C under plasma catalysis treatment. When the H2/CO ratio is 3.2, the toluene removal rate and the tar removal rate are close to 100% and 97%, the CO conversion rate and CH4 selectivity approach about 88% and 97%, and the energy efficiencies in toluene removal and SGG methanation processes can reach 9.7 g/(kW·h) and 17.3 mol/(kW·h). Both the high H2/CO ratio and the H2O addition can promote toluene removal and SGG methanation, and reduce the amount of carbon deposition but increase its graphitized degree. Moreover, high H2/CO ratio can rise the heating value of SGG and achieve higher energy efficiencies in both toluene removal and SGG methanation processes, while the H2O addition is difficult to obtain high CH4 selectivity to increase the heating value and is not conducive to the promotion of energy efficiency in SGG methanation process. In addition, for these two simultaneous processes, the SGG methanation process exerts a significant inhibiting effect on toluene removal, but process of toluene removal has less influence on the other process due to the lower concentration of toluene.
-
Key words:
- biomass gasification /
- tar removal /
- methanation /
- nonthermal plasma /
- catalysis
-
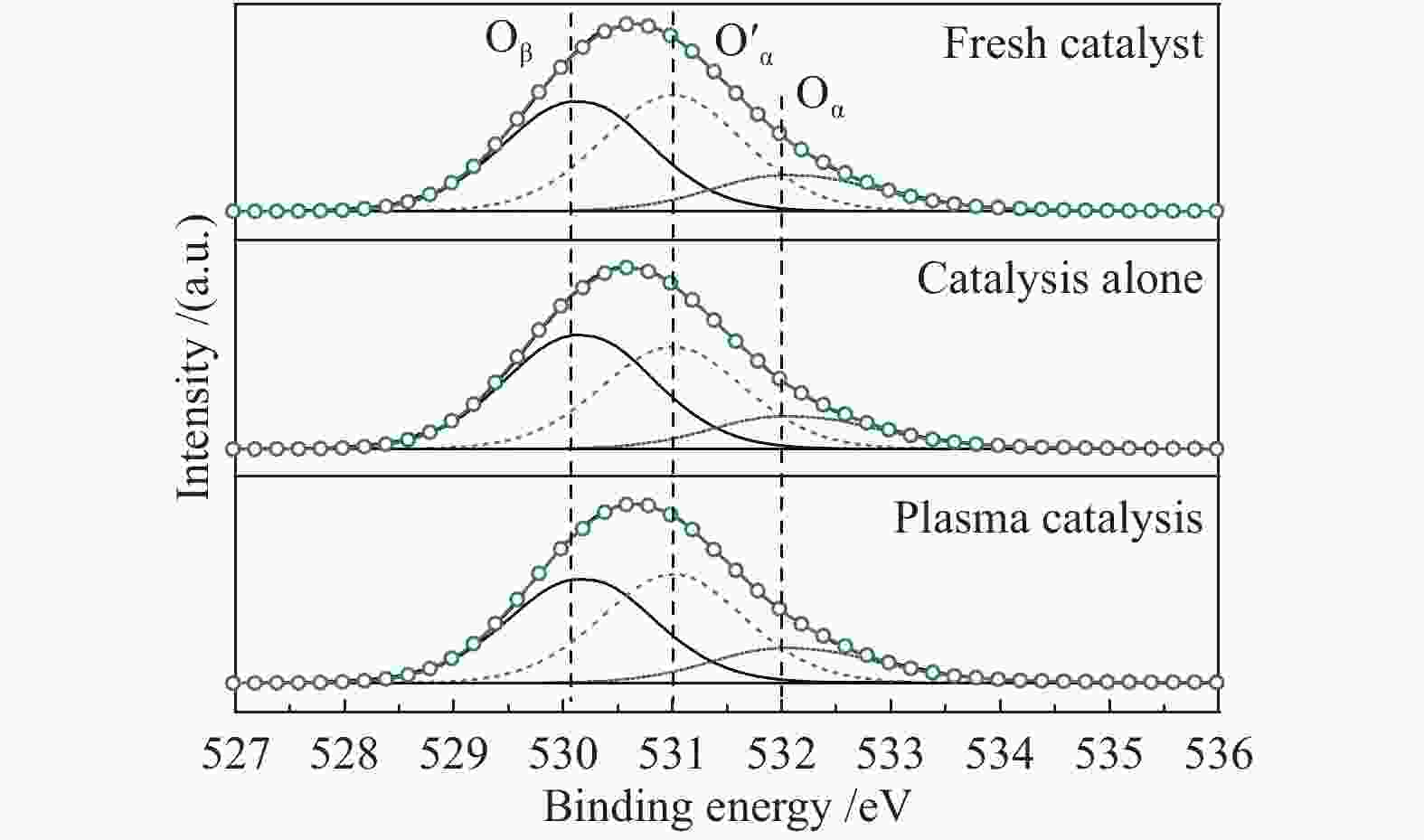
表 1 不同工况反应后催化剂的表面氧物种
Table 1 Surface oxygen species derived from O 1s spectra
Proces Oxygen concentration/% O′α/Oβ ratio Oβ O′α Oα Fresh catalyst 41.45 42.72 15.83 1.03 Catalyst alone 45.49 39.39 15.13 0.86 Plasma catalysis 41.53 42.40 16.07 1.02 表 2 不同工况下反应60 min后的催化剂积炭量
Table 2 Amount of carbon deposition on the catalysts reacted under different conditions for 60 min
Process Carbon deposition w/% SGG 1.38 H2/CO = 1.5 0.57 H2/CO = 2.2 0.16 H2/CO = 3.2 0.10 10 % H2O addition 0.17 20 % H2O addition 0.12 30 % H2O addition 0.03 表 3 400 ℃不同工况下的SGG 热值及能量效率
Table 3 LHV of SGG and energy efficiencies under different conditions operated at 400 ℃
H2/CO ratio H2O addition/% 0.8 1.5 2.2 3.2 10 20 30 QLHV Inlet/(MJ·m−3) 4.43 5.01 5.70 6.34 4.43 4.43 4.43 Outlet/(MJ·m−3) 4.06 5.06 6.01 7.49 3.97 3.83 3.72 Growth rate/% −8.4 0.1 5.4 18.1 −0.2 −13.5 −16.0 Energy efficiency Etoluene/(g·(kW·h)−1) 8.8 9.4 9.6 9.7 9.4 9.4 9.3 $ E_{{\rm{CH_4}}} $/(mol·(kW·h)−1) 4.3 9.6 13.7 17.3 6.1 5.0 3.1 -
[1] KUMAR A, DEMIREL Y, JONES D D, HANNA M A. Optimization and economic evaluation of industrial gas production and combined heat and power generation from gasification of corn stover and distillers grains[J]. Bioresour Technol,2010,101(10):3696−3701. doi: 10.1016/j.biortech.2009.12.103 [2] LEIBBRANDT N H, ABOYADE A O, KNOETZE J H, GÖRGENS J F. Process efficiency of biofuel production via gasification and Fischer-Tropsch synthesis[J]. Fuel,2013,109(7):484−492. [3] KOPYSCINSKI J, SCHILDHAUER T J, BIOLLAZ S M A. Production of synthetic natural gas (SNG) from coal and dry biomass – A technology review from 1950 to 2009[J]. Fuel,2010,89(8):1763−1783. doi: 10.1016/j.fuel.2010.01.027 [4] LIU Y, ZHU L, WANG X, YIN S, LENG F, ZHANG F, LIN H, WANG S. Catalytic methanation of syngas over Ni-based catalysts with different supports[J]. Chin J Chem Eng,2017,25(5):602−608. doi: 10.1016/j.cjche.2016.10.019 [5] 武宏香, 赵增立, 王小波, 郑安庆, 李海滨, 何方. 生物质气化制备合成天然气技术的研究进展[J]. 化工进展,2013,32(01):83−90,113.WU Hong-xiang, ZHAO Zeng-li, WANG Xiao-bo, ZHENG An-qing, LI Hai-bin, HE Fang. Technical development on synthetic natural gas production from biomass[J]. Chem Ind Eng Prog,2013,32(01):83−90,113. [6] LI C S, SUZUKI K. Tar property, analysis, reforming mechanism and model for biomass gasification-An overview[J]. Renewable Sustainable Energy Rev,2009,13(3):594−604. doi: 10.1016/j.rser.2008.01.009 [7] ANIS S, ZAINAL Z A. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review[J]. Renewable Sustable Energy Rev,2011,15(5):2355−2377. doi: 10.1016/j.rser.2011.02.018 [8] CHEN Y, LUO Y H, WU W G, SU Y. Experimental investigation on tar formation and destruction in a lab-scale two-stage reactor[J]. Energy Fuels,2009,23(9):4659−4667. doi: 10.1021/ef900623n [9] SHEN Y, YOSHIKAWA K. Recent progresses in catalytic tar elimination during biomass gasification or pyrolysis-A review[J]. Renewable Sustable Energy Rev,2013,21:371−392. doi: 10.1016/j.rser.2012.12.062 [10] KIENBERGER T, ZUBER C, NOVOSEL K, BAUMHAKL C, KARL J. Desulfurization and in situ tar reduction within catalytic methanation of biogenous synthesis gas[J]. Fuel,2013,107:102−112. doi: 10.1016/j.fuel.2013.01.061 [11] ZHANG J, WANG G, XU S. Simultaneous tar reforming and syngas methanation for bio-substitute natural gas[J]. Ind Eng Chem Res,2018,57(32):10905−10914. doi: 10.1021/acs.iecr.8b02085 [12] TATAROVA E, BUNDALESKA N, SARRETTE J P, FERREIRA C M. Plasmas for environmental issues: from hydrogen production to 2D materials assembly[J]. Plasma Sources Sci Technol,2014,23(6):063002. doi: 10.1088/0963-0252/23/6/063002 [13] OBRADOVIĆ B M, SRETENOVIĆ G B, KURAICA M M. A dual-use of DBD plasma for simultaneous NOx and SO2 removal from coal-combustion flue gas[J]. J Hazard Mater,2011,185(2/3):1280−1286. [14] CHUNG W C, PAN K L, LEE H M, CHANG M B. Dry reforming of methane with dielectric barrier discharge and ferroelectric packed-bed reactors[J]. Energy Fuels,2016,28(12):7621−7631. [15] TU X, WHITEHEAD J C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: Understanding the synergistic effect at low temperature[J]. Appl Catal B: Environ,2012,125(Supplement C):439−448. [16] XU B, XIE J, ZHAN H, YIN X, WU C, LIU H. Removal of toluene as a biomass tar surrogate in a catalytic nonthermal plasma process[J]. Energy Fuels,2018,32(10):10709−10719. doi: 10.1021/acs.energyfuels.8b02444 [17] LIU S Y, MEI D H, NAHIL M A, GADKARI S, GU S, WILLIAMS P T, TU X. Hybrid plasma-catalytic steam reforming of toluene as a biomass tar model compound over Ni/Al2O3 catalysts[J]. Fuel Process Technol,2017,166:269−275. doi: 10.1016/j.fuproc.2017.06.001 [18] LIU L, WANG Q, AHMAD S, YANG X, JI M, SUN Y. Steam reforming of toluene as model biomass tar to H2-rich syngas in a DBD plasma-catalytic system[J]. J Energy Inst,2018,91(6):927−939. doi: 10.1016/j.joei.2017.09.003 [19] 徐彬, 谢建军, 袁洪友, 阴秀丽, 吴创之. 填充床介质阻挡放电脱除气化燃气中苯的研究[J]. 燃料化学学报,2019,47(4):493−503.XU Bin, XIE Jian-jun, YUAN Hong-you, YIN Xiu-li, WU Chuang-zhi. Experimental study of benzene removal in fuel gas in a packed-bed dielectric barrier discharge reactor[J]. J Fuel Chem Technol,2019,47(4):493−503. [20] 董新新, 金保昇, 王妍艳, 牛淼淼. Ni/γ-Al2O3甲烷化催化剂提高生物质气化燃气低位热值的实验[J]. 东南大学学报(英文版),2017,33(4):448−456.DONG Xin-xin, JIN Bao-sheng, WANG Yan-yan, NIU Miao-miao. Experiments on Ni/γ-Al2O3 catalyst for improving lower heating value of biomass gasification fuel gas via methanation[J]. J Southeast Univ,2017,33(4):448−456. [21] NEYTS E C, BOGAERTS A. Understanding plasma catalysis through modelling and simulation-a review[J]. J Phys D Appl Phys,2014,47(22):224010. doi: 10.1088/0022-3727/47/22/224010 [22] WANG Q, YAN B H, JIN Y, CHENG Y. Dry reforming of methane in a dielectric barrier discharge reactor with Ni/Al2O3 catalyst: interaction of catalyst and plasma[J]. Energy Fuels,2009,23(8):4196−4201. doi: 10.1021/ef900286j [23] BLACKBEARD T, DEMIDYUK V, HILL S L, WHITEHEAD J C. The effect of temperature on the plasma-catalytic destruction of propane and propene: A comparison with thermal catalysis[J]. Plasma Chem Plasma P,2009,29(6):411−419. doi: 10.1007/s11090-009-9189-8 [24] LIU L N, WANG Q, SONG J W, AHMAD S, YANG X Y, SUN Y F. Plasma-assisted catalytic reforming of toluene to hydrogen rich syngas[J]. Catal Sci Technol,2017,7(18):4216−4231. doi: 10.1039/C7CY00970D [25] ANG M L, OEMAR U, KATHIRASER Y, SAW E T, LEW C H K, DU Y, BORGNA A, KAWI S. High-temperature water–gas shift reaction over Ni/xK/CeO2 catalysts: Suppression of methanation via formation of bridging carbonyls[J]. J Catal,2015,329:130−143. doi: 10.1016/j.jcat.2015.04.031 [26] LIU F D, HONG H, YUN D, ZHANG C B. Effect of manganese substitution on the structure and activity of iron titanate catalyst for the selective catalytic reduction of NO with NH3[J]. Appl Catal B: Environ,2009,93(1):3760−3769. [27] BITYURIN V A, FILIMONOVA E A, NAIDIS G V. Simulation of naphthalene conversion in biogas initiated by pulsed corona discharges[J]. IEEE Trans Plasma Sci,2009,37(6):911−919. doi: 10.1109/TPS.2009.2019756 [28] XU B, XIE J, YIN X, LIU H, SUN C G, WU C. Mechanisms of toluene removal in relation to the main components of biosyngas in a catalytic nonthermal plasma process[J]. Energy Fuels,2019,33(5):4287−4301. doi: 10.1021/acs.energyfuels.9b00273 [29] HUSSAIN I, JALIL A A, MAMAT C R, SIANG T J, RAHMAN A F A, AZAMI M S, ADNAN R H. New insights on the effect of the H2/CO ratio for enhancement of CO methanation over metal-free fibrous silica ZSM-5: Thermodynamic and mechanistic studies[J]. Energy Convers Manag,2019,199:112056. doi: 10.1016/j.enconman.2019.112056 [30] ABDELAZIZ A A, SETO T, ABDEL-SALAM M, OTANI Y. Influence of nitrogen excited species on the destruction of naphthalene in nitrogen and air using surface dielectric barrier discharge[J]. J Hazard Mater,2013,246–247:26−33. [31] SIMELL P A, HEPOLA J O, KRAUSE A O I. Effects of gasification gas components on tar and ammonia decomposition over hot gas cleanup catalysts[J]. Fuel,1997,76(12):1117−1127. doi: 10.1016/S0016-2361(97)00109-9 [32] TAN P H, ZHANG S L, YUE K T, HUANG F M, SHI Z J, ZHOU X H, GU Z N. Comparative Raman study of carbon nanotubes prepared by D. C. arc discharge and catalytic methods[J]. J Raman Spectrosc,1997,28(5):369−372. doi: 10.1002/(SICI)1097-4555(199705)28:5<369::AID-JRS107>3.0.CO;2-X [33] QIAN W Z, LIU T, WEI F, YUAN H Y. Quantitative Raman characterization of the mixed samples of the single and multi-wall carbon nanotubes[J]. Carbon,2003,41(9):1851−1854. doi: 10.1016/S0008-6223(03)00106-4 [34] ZHU F S, LI X D, ZHANG H, WU A J, YAN J H, NI M J, ZHANG H W, BUEKENS A. Destruction of toluene by rotating gliding arc discharge[J]. Fuel,2016,176:78−85. doi: 10.1016/j.fuel.2016.02.065 -




 下载:
下载: