Selective synthesis of dimethyl carbonate via the coupling reaction of CO2 and alcohols by the synergistic catalysis of silver sulfadiazine and superbase
-
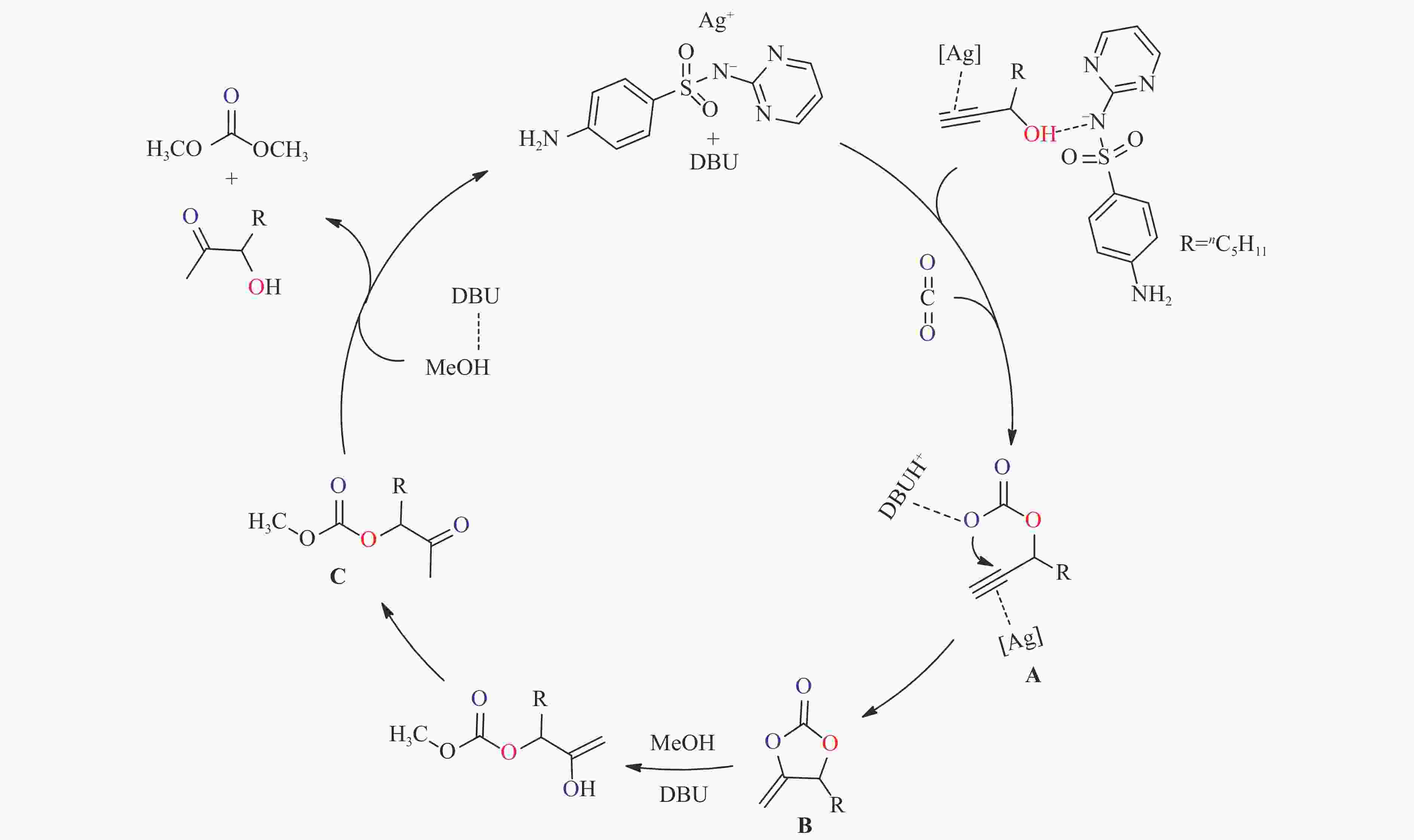
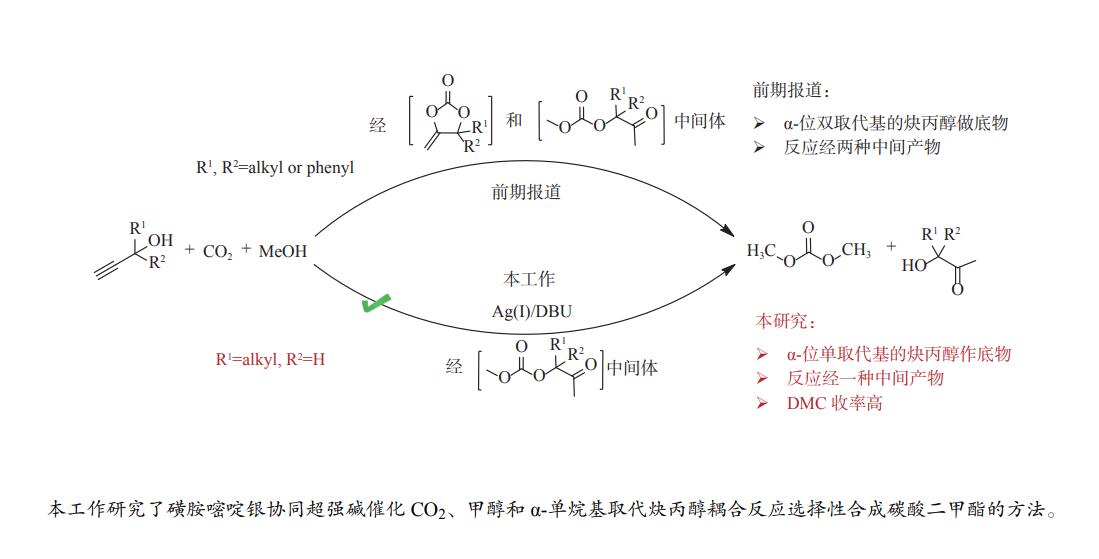
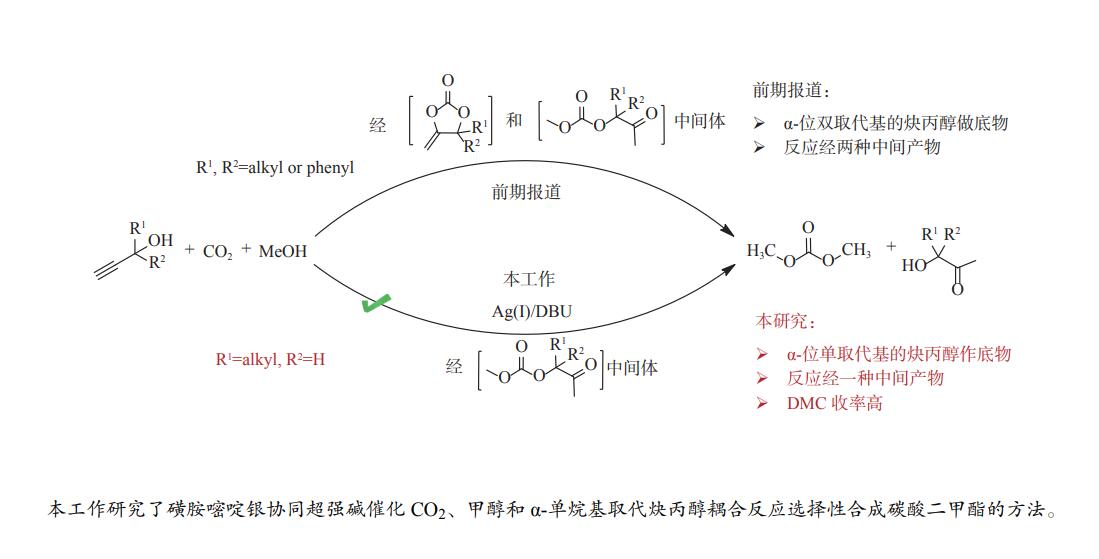
摘要: 甲醇、CO2和炔丙醇三组分耦合反应为合成绿色化学品碳酸二甲酯(DMC)提供了一种热力学有利新路径。本工作针对该方法中存在的DMC收率低、中间产物转化速率慢的问题,研究了炔烃衍生物α-单取代炔丙醇在耦合反应中对DMC收率及选择性的影响。发展了磺胺嘧啶银与超强碱协同催化串联反应策略,进一步提升了反应效率。考察了催化剂、共催化剂、催化剂用量、溶剂、温度、原料比、压力和时间等因素的影响规律。最优的条件下,DMC选择性达89.5%,收率55.6%。研究表明,炔丙醇的结构会显著影响反应进程,同时,磺胺嘧啶银与1,8-二氮杂二环十一碳-7-烯(DBU)的协同催化作用是高收率、高选择性获得DMC的关键因素。Abstract: The three-component coupling of methanol, CO2 and propargyl alcohol to manufacture dimethyl carbonate (DMC) provides a new, thermodynamic favorable and green chemical synthesis route. In this work, to overcome the problems of low DMC yield and slow conversion rate of intermediates, the synergistic catalytic strategy of silver sulfadiazine and superbase is developed to improve the reaction efficiency. The effect of various parameters i.e. catalyst and cocatalyst types, catalyst loading, solvent, temperature, proportion of raw materials, pressure and time on the coupling reactions is investigated in detail. Under the optimal conditions, the selectivity of DMC is 89.5% with the yield of 55.6%. And the effect of alkyne derivative α-monosubstituted propargyl alcohol on the efficiency and selectivity of DMC are also studied. The mechanism study shows that propargyl alcohols with different structures apparently affect the reaction process, and the synergistic catalysis of silver sulfadiazine/1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) is the reason for the high yield and selectivity of DMC.
-
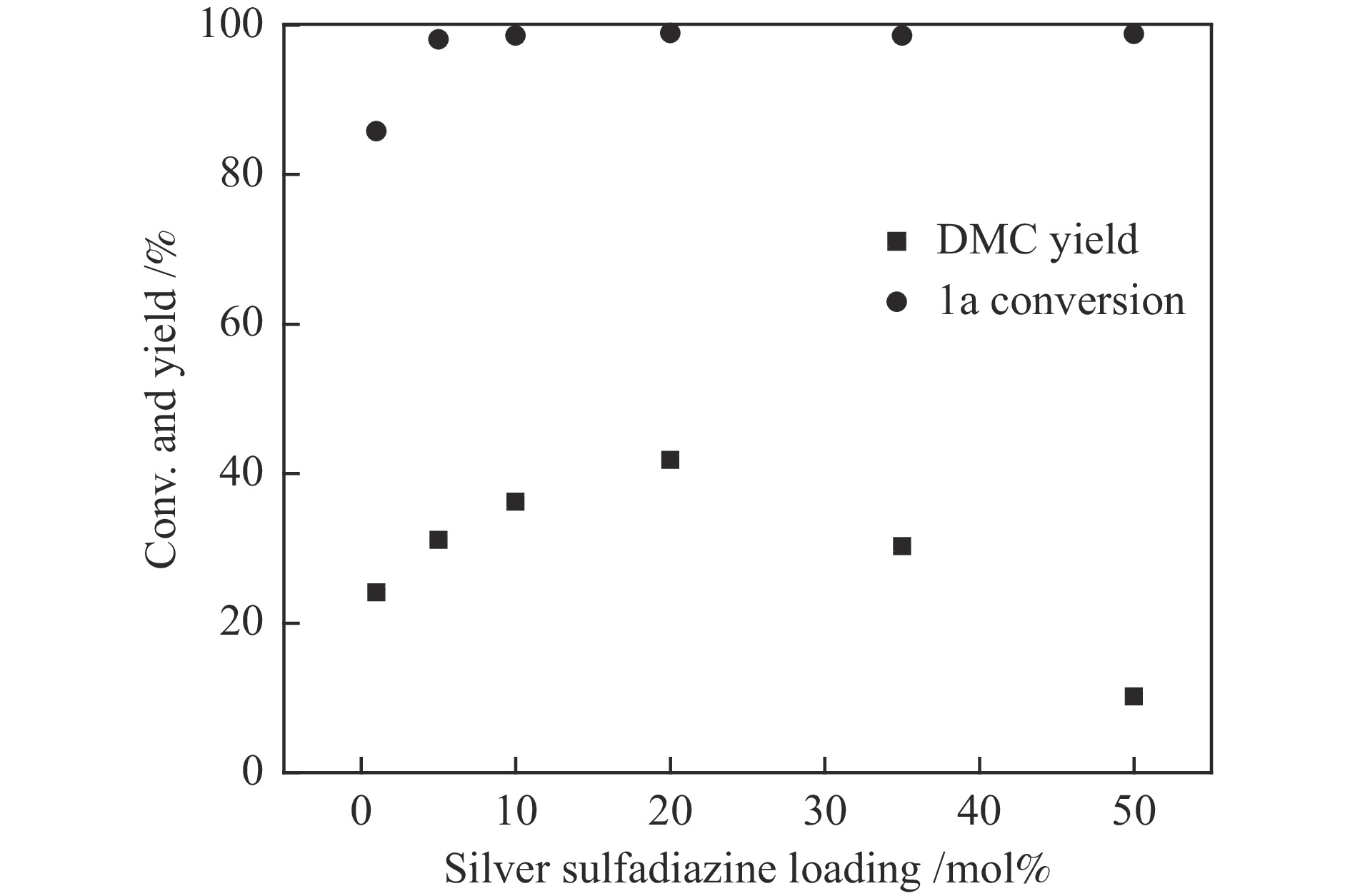
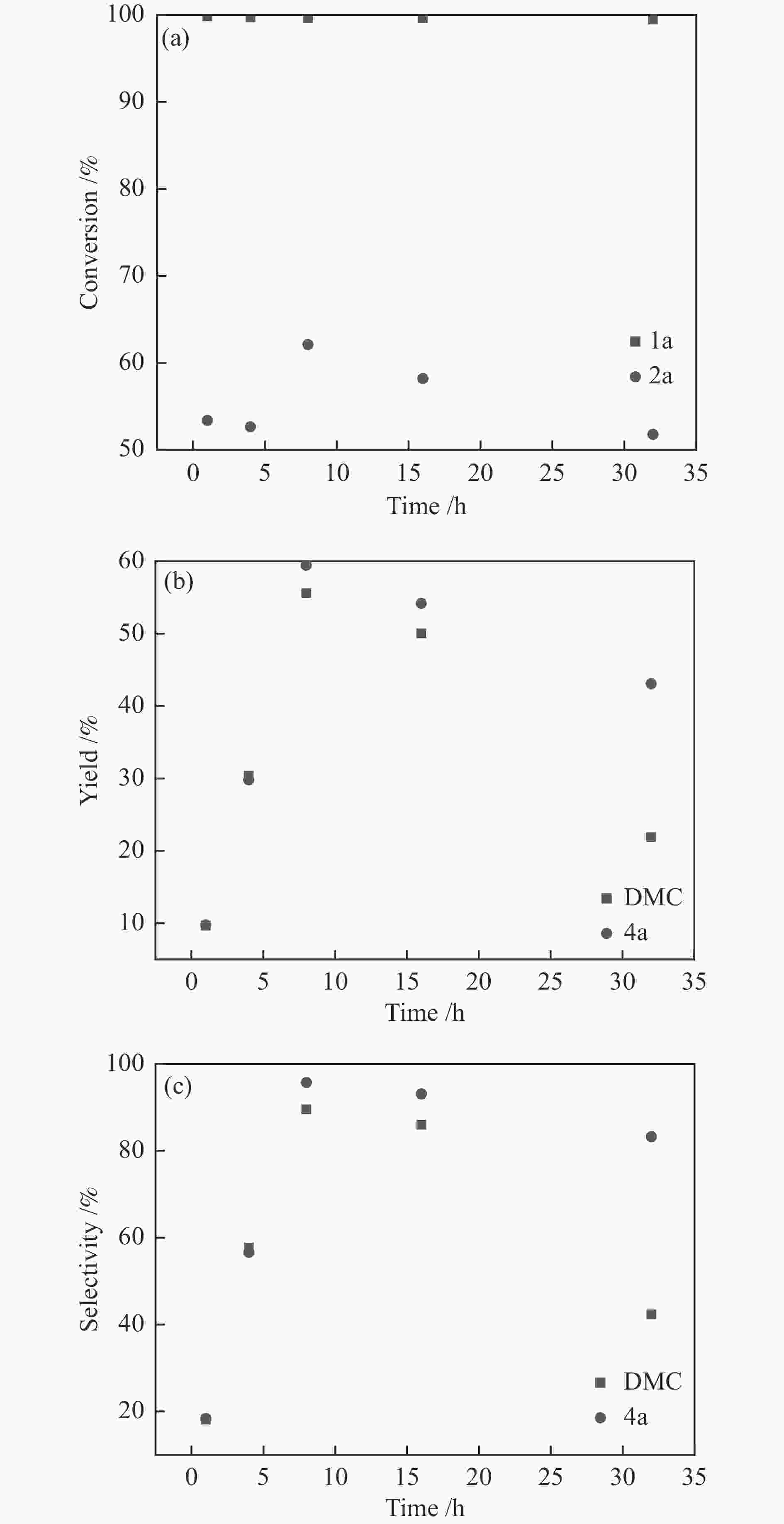
图 1 催化剂用量的影响
Figure 1 Catalyst loading investigation
Reaction condition: 1a (620 mg, 5 mmol), 2a (320 mg, 10 mmol), DBU (228 mg, 1.5 mmol), CO2 (5 MPa), 120 ℃, 8 h, the loading of silver sulfadiazine was calculated on the basis of 1a, the results were determined by GC using biphenyl as the internal standard
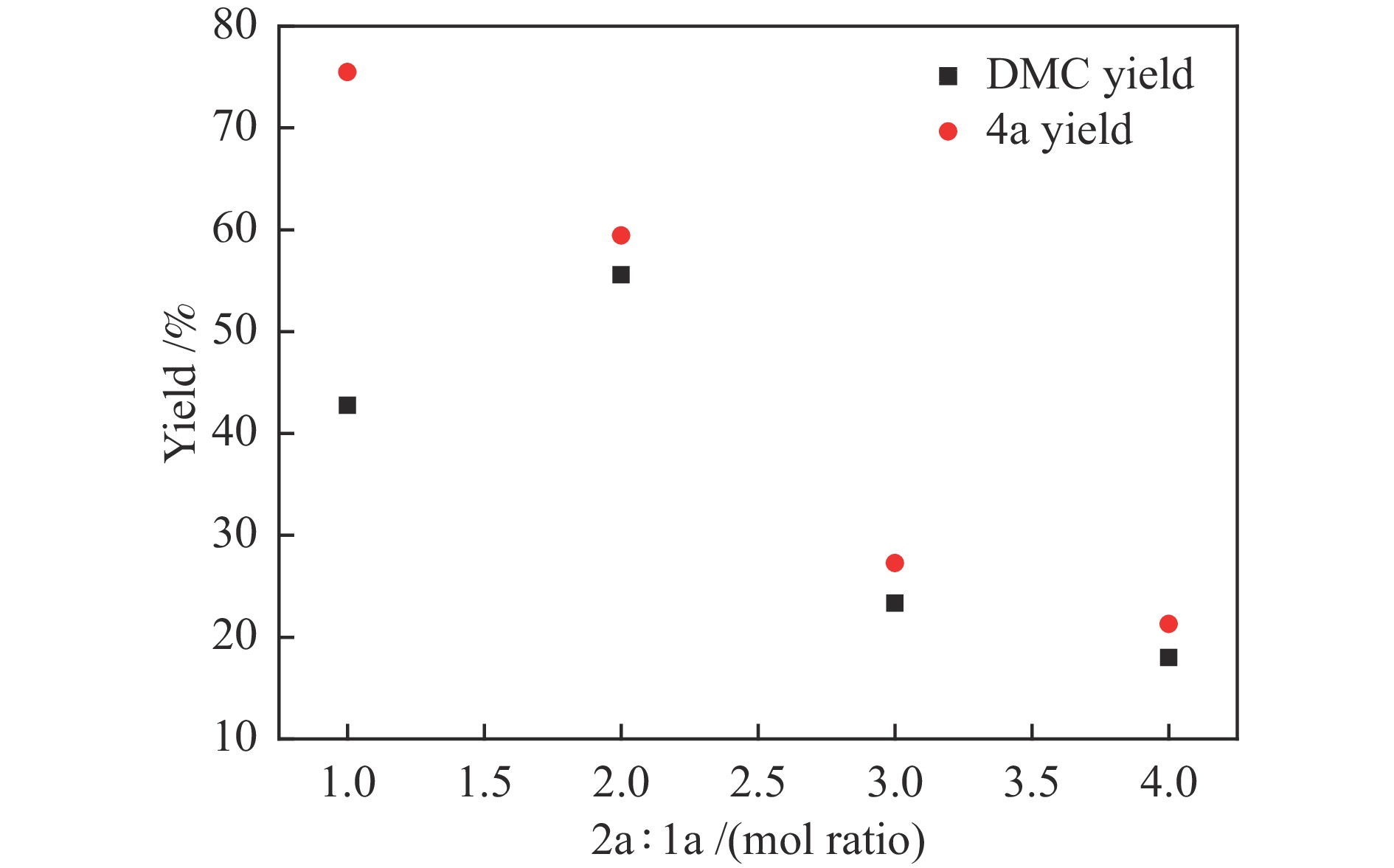
图 3 原料配比对反应的影响
Figure 3 Effect of raw material ratio on reaction
Reaction condition: 1a (620 mg, 5 mmol), 2a (160−640 mg, 10− 40 mmol), silver sulfadiazine (357 mg, 1 mmol), DBU (228 mg, 1.5 mmol), DMSO (1 mL), CO2 (5 MPa), 120 ℃, 8 h, the yield was determined by GC using biphenyl as the internal standard
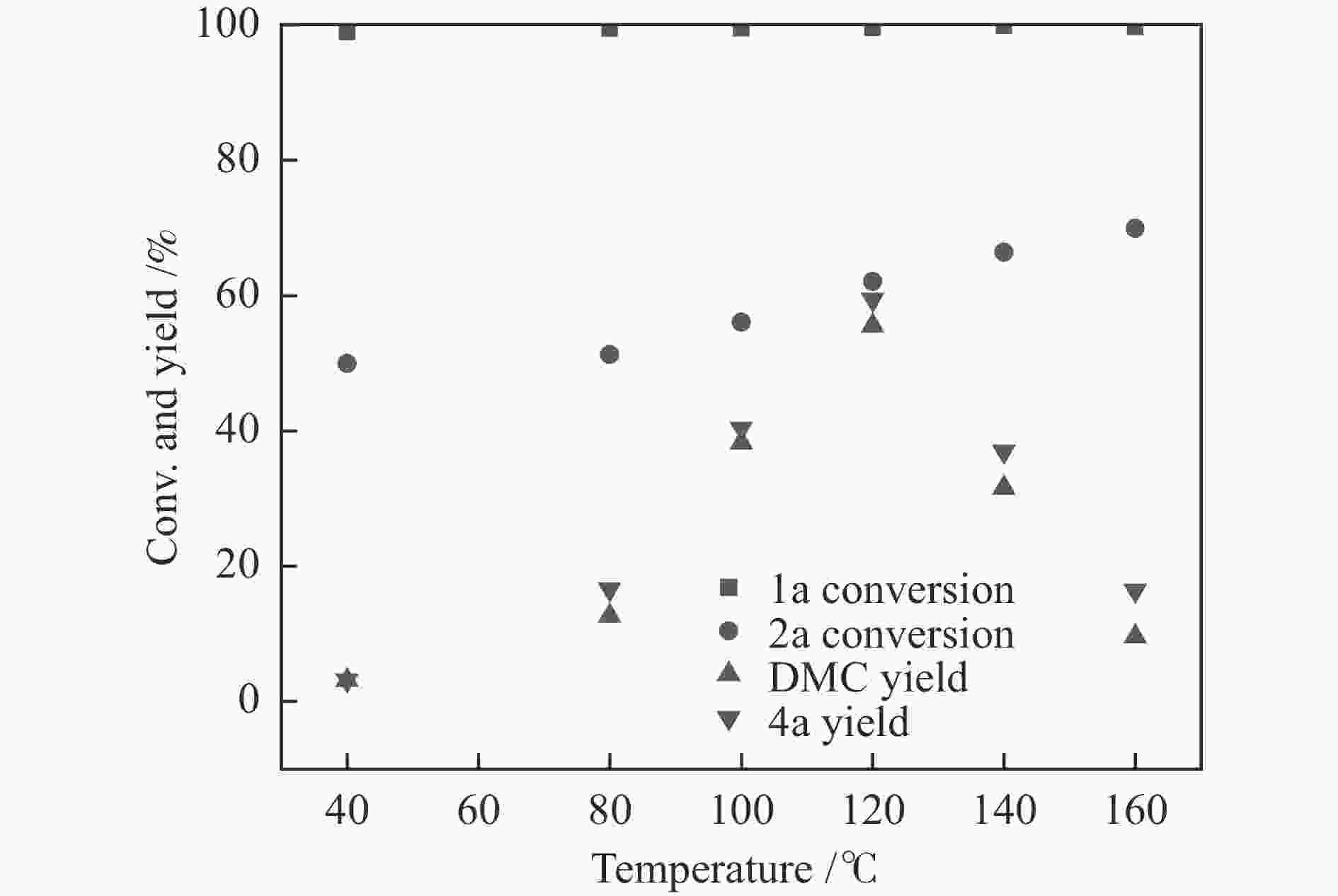
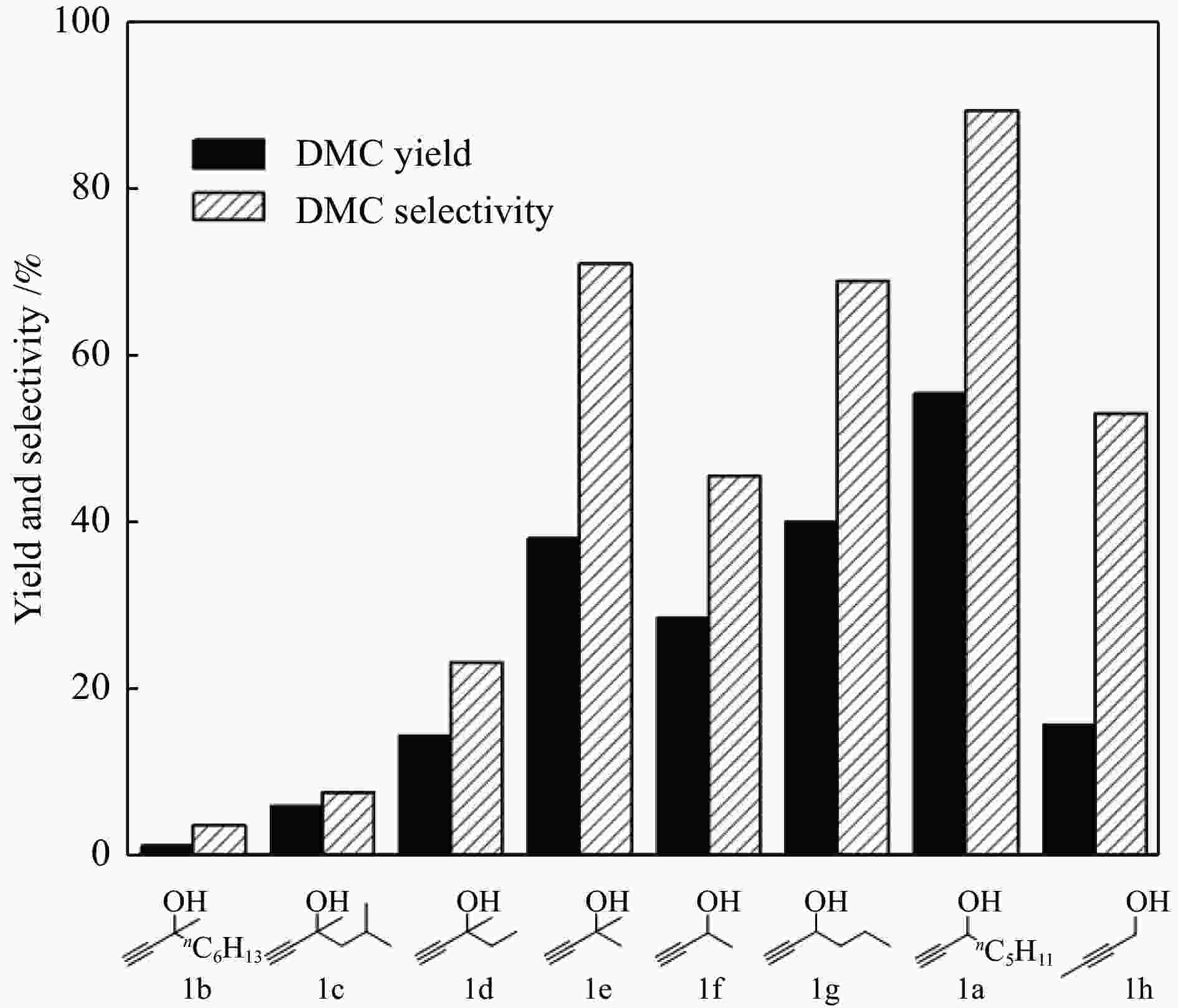
图 6 炔丙醇结构与DMC收率和选择性的关系
Figure 6 Relationship between propargyl alcohol structure and the yield/selectivity of DMC
Reaction condition: propargyl alcohol (5 mmol), methanol (320 mg, 10 mmol), silver sulfadiazine (357 mg, 1 mmol), DBU (228 mg, 1.5 mmol), DMSO (1 mL), CO2 (5 MPa), 120 ℃, 8 h, the results were determined by GC using biphenyl as the internal standard
表 1 催化剂性能
Table 1 Catalysts investigation

Entry Catalyst 1a conv./% 2a conv./% DMC yield/% 4a yield/% DMC sel./% 4a sel./% 1a − 0 0 0 0 − − 2 − 62.9 50.0 15.6 25.2 31.2 50.4 3 CoCO3 14.1 10.2 3.7 5.6 36.3 54.9 4 NiBr2 33.7 32.3 3.3 8.0 10.2 24.8 5 CuBr 96.5 48.6 35.4 36.3 72.9 74.7 6 ZnCl2 99.9 80.3 29.6 34.7 36.9 43.2 7 ZnBr2 98.7 77.5 33.1 39.0 42.7 54.6 8 ZnI2 98.3 40.6 17.8 27.1 43.8 42.5 9 Zn(OAc)2 99.2 58.6 30.4 41.6 51.9 71.1 10 AgCl 98.1 39.2 30.5 35.1 77.7 89.6 11 AgI 99.5 38.1 16.7 23.7 43.8 62.1 12 AgOAc 99.0 44.0 34.8 41.5 79.1 94.2 13 Ag2SO4 99.3 64.9 26.2 35.9 40.5 55.4 14 Ag3PO4 96.3 41.9 27.7 36.9 66.2 88.1 15 AgVO3 53.6 35.8 20.0 27.7 56.1 77.5 16 CF3SO3Ag 95.7 62.8 10.1 23.1 16.0 36.9 17 C7H5AgO2 97.2 38.9 25.2 29.7 64.8 76.3 18 Ag2CO3 76.8 61.9 12.2 46.9 19.7 75.9 19 Ag2O 98.8 53.8 40.4 46.3 75.1 86.1 20 silver sulfadiazine 99.9 53.1 41.8 42.6 78.7 80.4 Reaction condition: 1a (620 mg, 5 mmol), 2a (320 mg, 10 mmol), catalyst (1 mmol), DBU (228 mg, 1.5 mmol), CO2 (5 MPa), 120 ℃, 8 h, a: In the absence of any catalyst and cocatalyst, the results were determined by GC using biphenyl as the internal standard, conversion (conv.), selectivity (sel.) 表 2 共催化剂性能
Table 2 Co-catalyst investigation
Entry Co-catalyst 1a conv./% 2a conv./% DMC
yield/%4a yield/% DMC sel./% 4a sel./% 1a − 87.6 42.3 2.2 4.4 5.2 10.4 2 DBU 99.9 53.1 41.8 42.6 78.7 80.2 3 DBN 98.5 72.7 21.9 34.8 30.1 47.9 4 TMG 99.7 65.0 17.5 18.5 26.9 28.5 5 TBD 98.7 72.6 2.2 3.5 3.0 4.8 6 TBAB 100.0 88.2 3.2 3.4 3.6 3.9 7 TEAC 99.5 78.7 4.7 7.2 6.0 9.1 8 TBAB/DBU 99.7 73.6 22.8 35.5 31.0 48.2 9 PPh3/DBU 100.0 49.2 2.8 6.4 5.7 13.0 Reaction condition: 1a (620 mg, 5 mmol), 2a (320 mg, 10 mmol), silver sulfadiazine (1 mmol), cocatalyst (1.5 mmol), CO2 (5 MPa), 120 ℃, 8 h, a: only silver sulfadiazine was used, the results were determined by GC using biphenyl as the internal standard 表 3 溶剂对反应的影响
Table 3 Solvent effect on the reaction
Entry Solvent 1a conv./% 2a conv./% DMC yield/% 4a yield/% DMC sel./% 4a sel./% 1 Blank 99.9 53.1 41.8 42.6 78.7 80.4 2 DMSO 99.6 62.1 55.6 59.4 89.5 95.7 3 DMF 99.5 53.9 35.7 39.0 66.3 72.4 4 CH3CN 99.5 58.2 34.4 37.8 59.1 65.0 5 EtOAc 99.4 48.7 24.4 33.3 50.0 68.4 6 PhCH3 98.0 51.3 37.3 41.7 72.8 81.3 7 tetrahydrofuran 99.6 49.2 29.2 35.2 59.3 71.5 Reaction condition: 1a (620 mg, 5 mmol), 2a (320 mg, 10 mmol), silver sulfadiazine (357 mg, 1 mmol), DBU (228 mg, 1.5 mmol), solvent (1 mL), CO2 (5 MPa), 120 ℃, 8 h, the results were determined by GC using biphenyl as the internal standard -
[1] SONG Q W, ZHOU Z H, HE L N. Efficient, selective and sustainable catalysis of carbon dioxide[J]. Green Chem,2017,19(16):3707−3728. doi: 10.1039/C7GC00199A [2] LU M, ZHANG M, LIU J, CHEN Y, LIAO J P, YANG M Y, CAI Y P, LI S L, LAN Y Q. Covalent organic framework based functional materials: important catalysts for efficient CO2 utilization[J]. Angew Chem Int Ed,2022,61(15):e202200003. [3] HE X, QIU L Q, WANG W J, CHEN K H, HE L N. Photocarboxylation with CO2: an appealing and sustainable strategy for CO2 fixation[J]. Green Chem,2020,22(21):7301−7320. doi: 10.1039/D0GC02743J [4] AOMCHAD V, CRISTOFOL A, DELLA F, LIMBURG B, DELIA V, KLEIJ A W. Recent progress in the catalytic transformation of carbon dioxide into biosourced organic carbonates[J]. Green Chem,2021,23(3):1077−1113. doi: 10.1039/D0GC03824E [5] BURKART M D, HAZARI N, TWAY C L, ZEITLER E L. Opportunities and challenges for catalysis in carbon dioxide utilization[J]. ACS Catal,2019,9(9):7937−7956. doi: 10.1021/acscatal.9b02113 [6] TAN H Z, WANG Z Q, XU Z N, SUN J, XU Y P, CHEN Q S, CHEN Y, GUO G C. Review on the synthesis of dimethyl carbonate[J]. Catal Today,2018,316:2−12. doi: 10.1016/j.cattod.2018.02.021 [7] KUMAR P, SRIVASTAVA V C, STANGAR U L, MUSIC B, MISHRA I M, MENG Y. Recent progress in dimethyl carbonate synthesis using different feedstock and techniques in the presence of heterogeneous catalysts[J]. Catal Rev,2019,63(3):363−421. [8] TAMBOLI A H, CHAUGULE A A, KIM H. Catalytic developments in the direct dimethyl carbonate synthesis from carbon dioxide and methanol[J]. Chem Eng J,2017,323:530−544. doi: 10.1016/j.cej.2017.04.112 [9] BALLIVET D, JERPHAGNON T, LIGABUE R, PLASSERAUD L, POINSOT D. The role of distannoxanes in the synthesis of dimethyl carbonate from carbon dioxide[J]. Appl Catal A: Gen,2003,255(1):93−99. doi: 10.1016/S0926-860X(03)00647-1 [10] HONDA M, TAMURA M, NAKAGAWA Y, SONEHARA S, SUZUKI K, FUJIMOTO K, TOMISHIGE K. Ceria-catalyzed conversion of carbon dioxide into dimethyl carbonate with 2-cyanopyridine[J]. ChemSusChem,2013,6(8):1341−1346. doi: 10.1002/cssc.201300229 [11] HE H, QI C, HU X, GUAN Y, JIANG H. Efficient synthesis of tertiary α-hydroxy ketones through CO2-promoted region-selective hydration of propargylic alcohols[J]. Green Chem,2014,16(8):3729−3733. doi: 10.1039/C4GC00522H [12] ZHAO Y, YANG Z, YU B, ZHANG H, XU H, HAO L, HAN B, LIU Z. Task-specific ionic liquid and CO2-cocatalysed efficient hydration of propargylic alcohols to α-hydroxy ketones[J]. Chem Sci,2015,6(4):2297−2301. doi: 10.1039/C5SC00040H [13] ZHOU Z H, SONG Q W, HE L N. Silver(I)-promoted cascade reaction of propargylic alcohols, carbon dioxide, and vicinal diols: thermodynamically favorable route to cyclic carbonates[J]. ACS Omega,2017,2(1):337−345. doi: 10.1021/acsomega.6b00407 [14] ZHOU H, ZHANG H, MU S, ZHANG W Z, REN W M, LU X B. Highly regio and stereoselective synthesis of cyclic carbonates from biomass-derived polyols via organocatalytic cascade reaction[J]. Green Chem,2019,21(23):6335−6341. doi: 10.1039/C9GC03013A [15] HU J, MA J, LU L, QIAN Q, ZHANG Z, XIE C, HAN B. Synthesis of asymmetrical organic carbonates using CO2 as a feedstock in AgCl/ionic liquid system at ambient conditions[J]. ChemSusChem,2017,10(6):1292−1297. doi: 10.1002/cssc.201601773 [16] 张乾霞, 刘平, 张侃, 韩丽华, 宋清文. 多组分串联策略固定CO2制碳酸二甲酯和α-羟基酮[J]. 科学通报,2020,65(31):3429−3437. doi: 10.1360/TB-2020-0351ZHANG Qian-xia, LIU Ping, ZHANG Kan, HAN Li-hua, SONG Qing-wen. Multicomponent cascade strategy of CO2 fixation for synthesis of dimethyl carbonate and α-hydroxy ketone[J]. Chin Sci Bull,2020,65(31):3429−3437. doi: 10.1360/TB-2020-0351 [17] SONG Q W, CHEN W Q, MA R, YU A, LI Q Y, CHANG Y, HE L N. Bifunctional silver(I) complex-catalyzed CO2 conversion at ambient conditions: synthesis of α-methylene cyclic carbonates and derivatives[J]. ChemSusChem,2015,8(5):821−827. doi: 10.1002/cssc.201402921 [18] YUAN Y, XIE Y, ZENG C, SONG D, CHAEMCHUEN S, CHEN C, VERPOORT F. A simple and robust AgI/KOAc catalytic system for the carboxylative assembly of propargyl alcohols and carbon dioxide at atmospheric pressure[J]. Catal Sci Technol,2017,7(14):2935−2939. doi: 10.1039/C7CY00696A [19] LI J Y, HAN L H, XU Q C, SONG Q W, LIU P, ZHANG K. Cascade strategy for atmospheric pressure CO2 fixation to cyclic carbonates via silver sulfadiazine and Et4NBr synergistic catalysis[J]. ACS Sustainable Chem Eng,2019,7(3):3378−3388. doi: 10.1021/acssuschemeng.8b05579 [20] CERVANTES R A, SAXL T, STEIN P M, RUDOLPH M, ROMINGER F, ASIRI A M, HASHMI A S. Expanded ring NHC silver carboxylate complexes as efficient and reusable catalysts for the carboxylative cyclization of unsubstituted propargylic derivatives[J]. ChemSusChem,2021,14(11):2367−2374. doi: 10.1002/cssc.202002822 [21] MCCORMACK A C, OFERRALL R A, ODONOGHU A C, RAO S N. Protonated benzofuran, anthracene, naphthalene, benzene, ethene, and ethyne: measurements and estimates of pKa and pKR[J]. J Am Chem Soc,2002,124(29):8575−8583. doi: 10.1021/ja012613x [22] CA N D, GABRIELE B, RUFFOLO G, VELTRI L, ZANETTA T, COSTA M. Effective Guanidine-catalyzed synthesis of carbonate and carbamate derivatives from propargyl alcohols in supercritical carbon dioxide[J]. Adv Synth Catal,2011,353(1):133−146. doi: 10.1002/adsc.201000607 [23] ZHOU Z H, SONG Q W, XIE J N, MA R, HE L N. Silver(I)-catalyzed three-component reaction of propargylic alcohols, carbon dioxide and monohydric alcohols: Thermodynamically feasible access to β-oxopropyl carbonates[J]. Chem-Asian J,2016,11(14):2065−2071. [24] SEKINE K, YAMADA T. Silver-catalyzed carboxylation[J]. Chem Soc Rev,2016,45(16):4524−4532. doi: 10.1039/C5CS00895F -
 2022-S016_辅助材料_燃料化学学报.docx
2022-S016_辅助材料_燃料化学学报.docx
-




 下载:
下载: