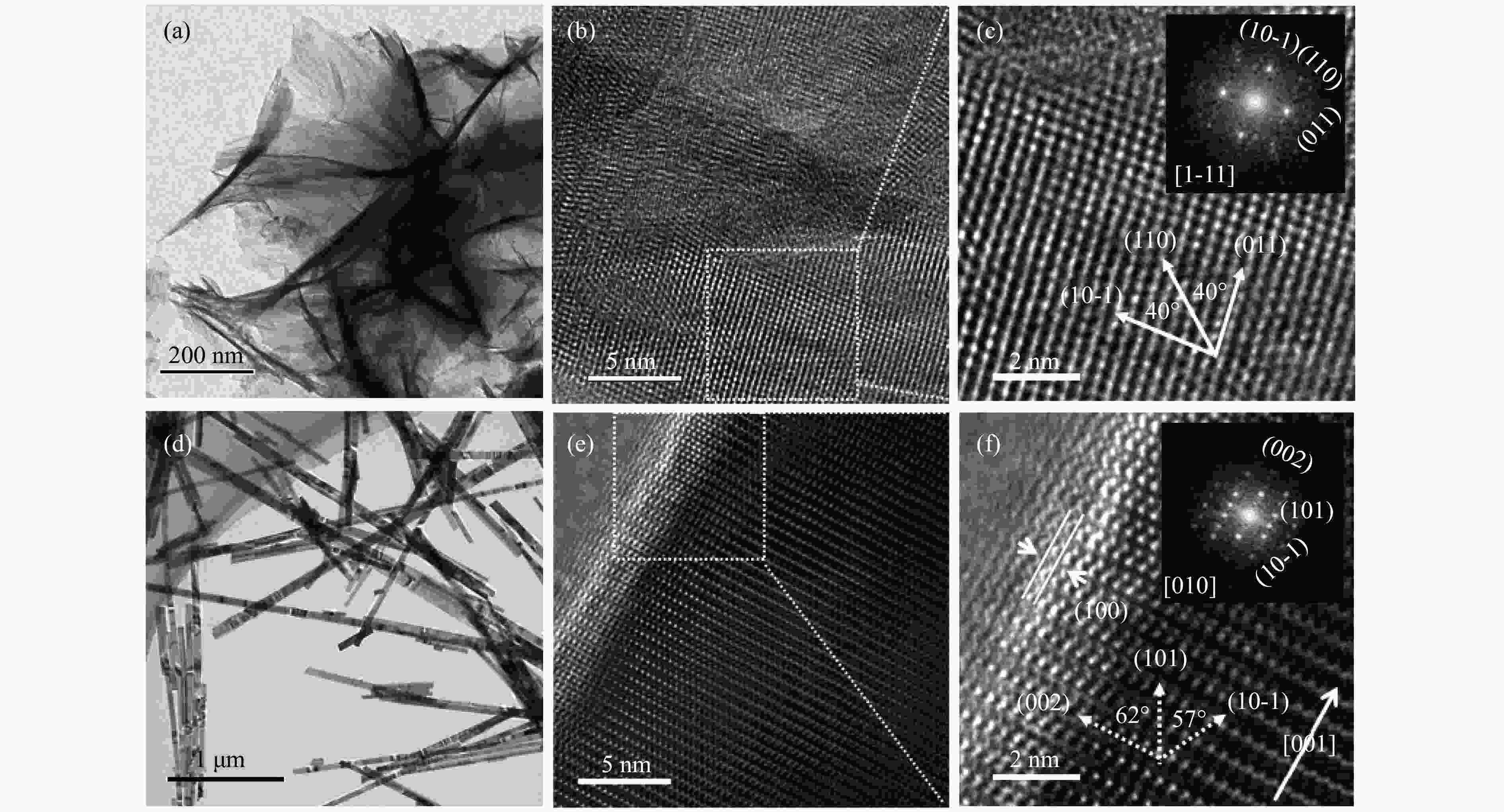
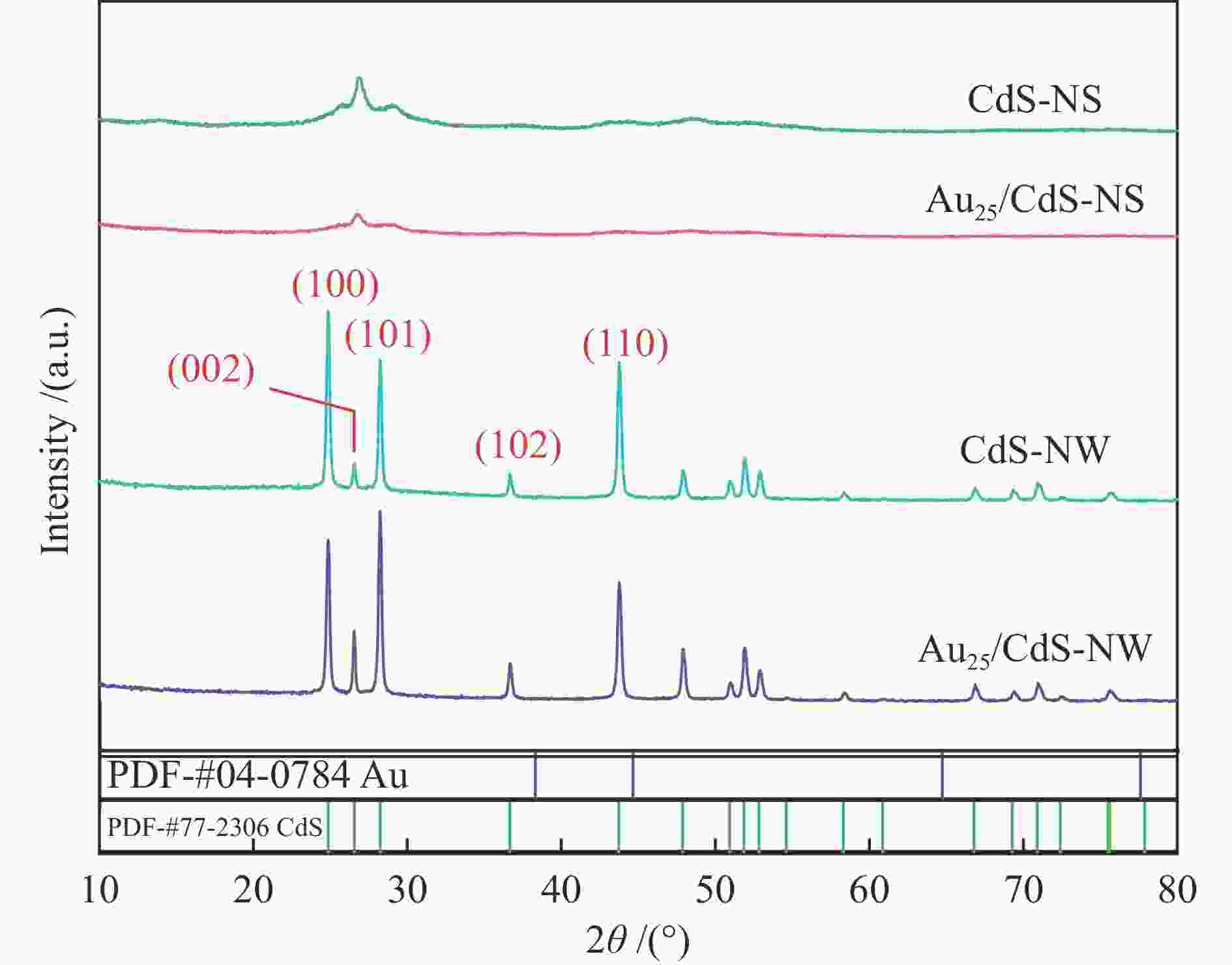
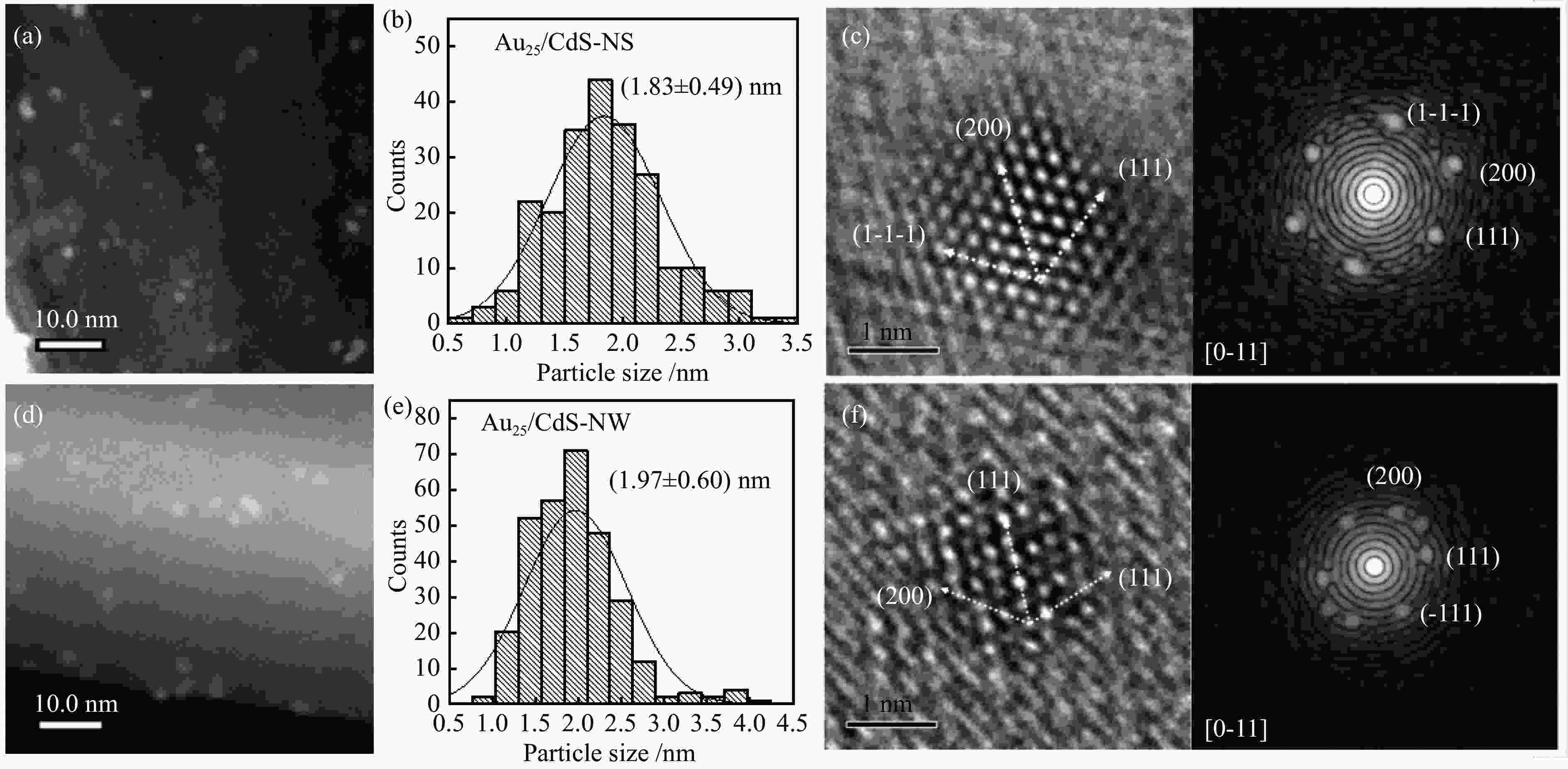
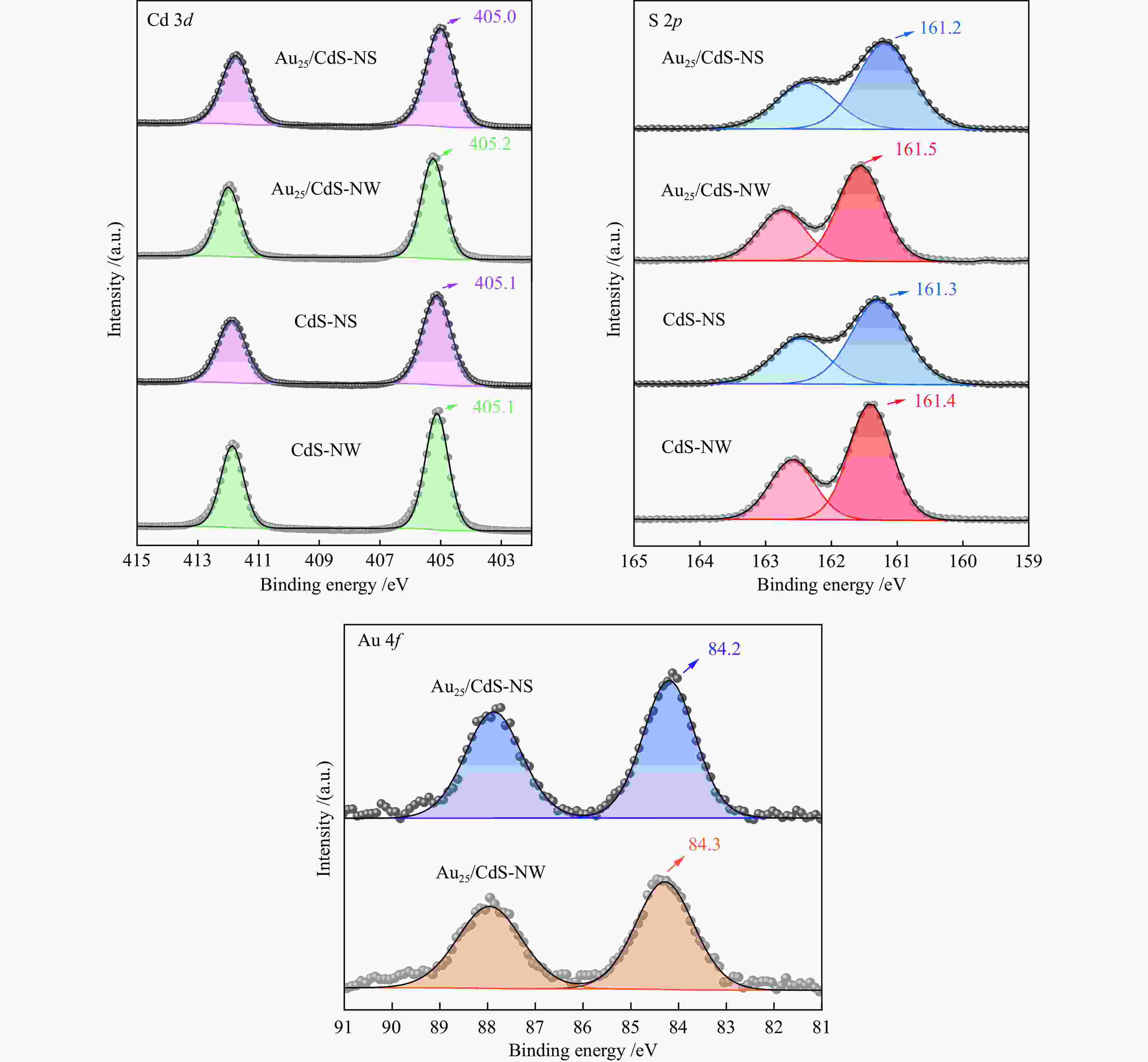
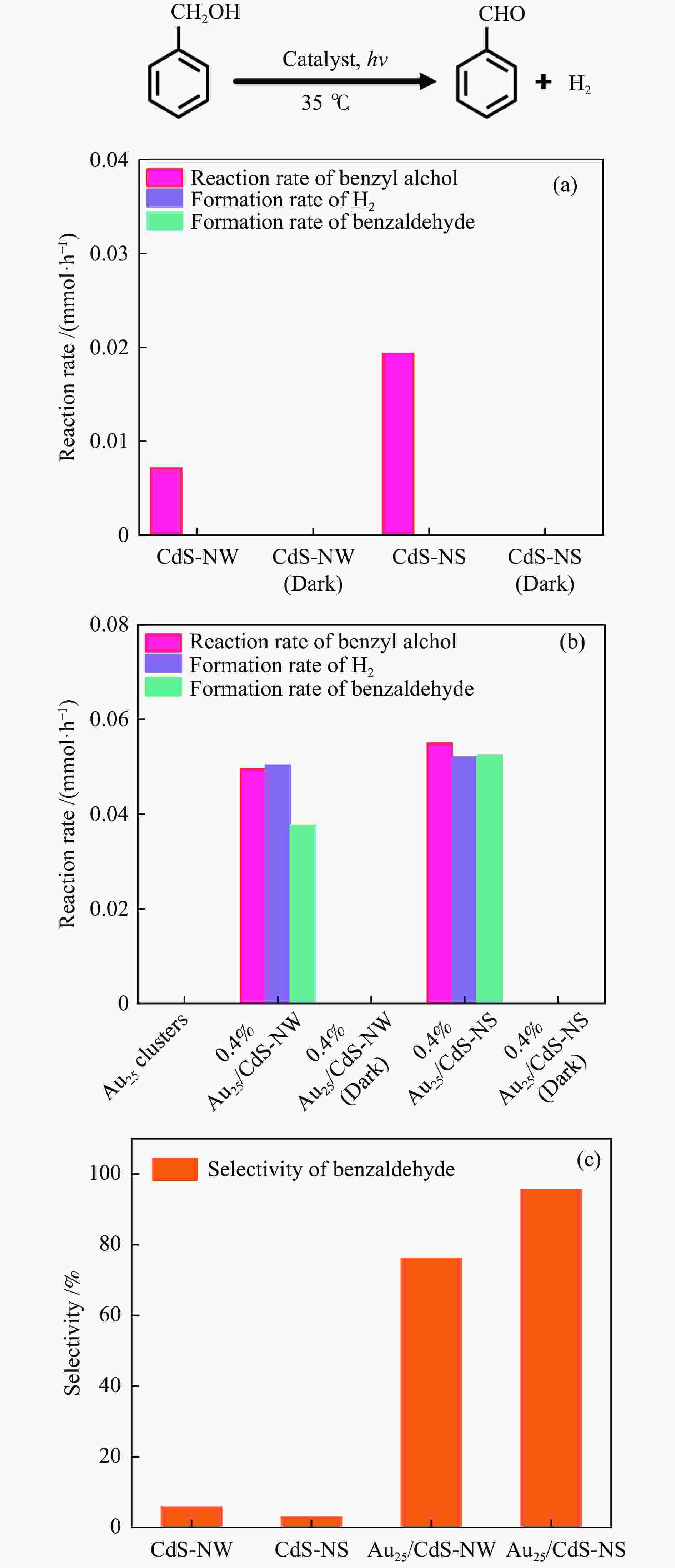
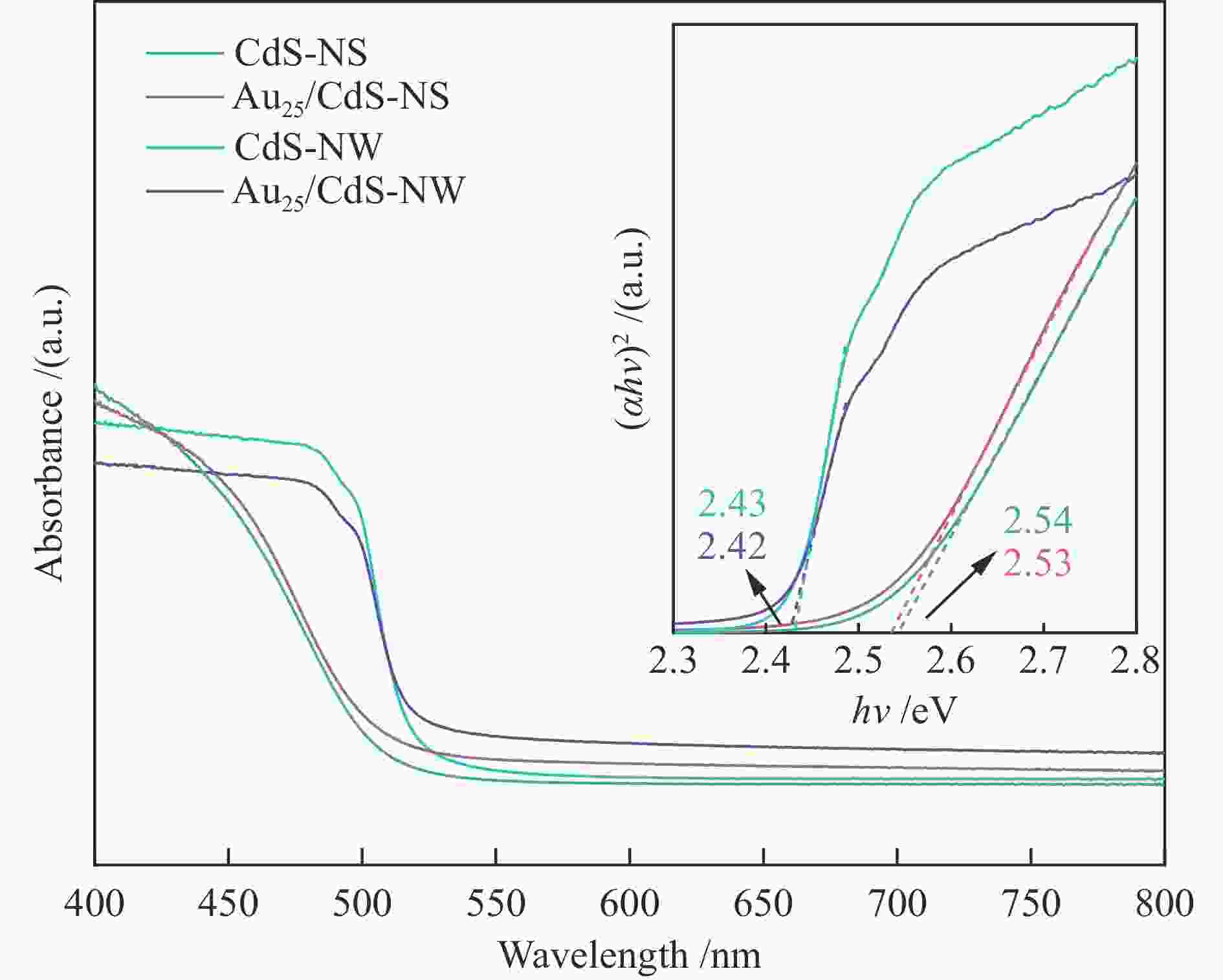
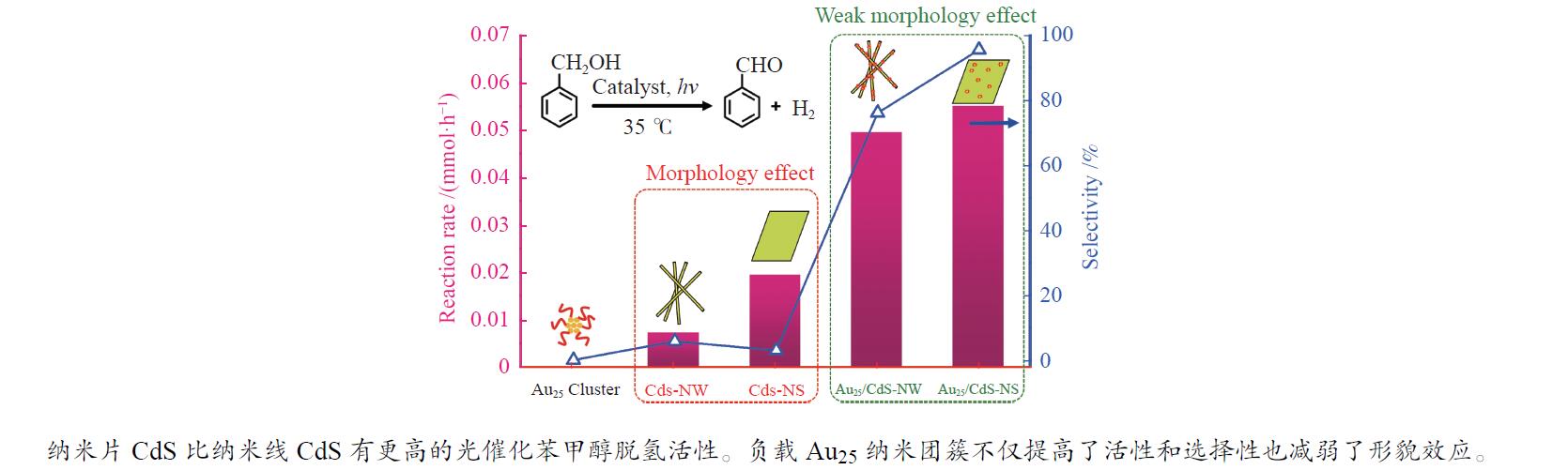
| [1] |
LUONG G K T, KU Y. Selective oxidation of benzyl alcohol in the aqueous phase by TiO2-based photocatalysts: A review[J]. Chem Eng Technol,2021,44:2178−2190. doi: 10.1002/ceat.202100321
|
| [2] |
PARRINO F, BELLARDITA M, GARCÍA-LÓPEZ E I, MARCÌ G, LODDO V, PALMISANO L. Heterogeneous photocatalysis for selective formation of high-value-added molecules: Some chemical and engineering aspects[J]. ACS Catal,2018,8:11191−11225. doi: 10.1021/acscatal.8b03093
|
| [3] |
TAN J, ZHANG W, LV Y H, XIA A L. Facile preparation of Mn-doped CeO2 Submicrorods by composite-hydroxide-salt-mediated approach and their magnetic property[J]. Mater Res,2013,16:689−694. doi: 10.1590/S1516-14392013005000040
|
| [4] |
LI D, HANEDA H, OHASHI N, SAITO N, HISHITA S. Morphological reform of ZnO particles induced by coupling with MOx (M=V, W, Ce) and the effects on photocatalytic activity[J]. Thin Solid Films,2005,486:20−23. doi: 10.1016/j.tsf.2004.11.237
|
| [5] |
LI R, TAO X, CHEN R, FAN F, LI C. Synergetic effect of dual co-catalysts on the activity of p-type Cu2O crystals with anisotropic facets[J]. Chem Eur J,2015,21:14337−14341. doi: 10.1002/chem.201502562
|
| [6] |
LI R, ZHANG F, WANG D, YANG J, LI M, ZHU J, ZHOU X, HAN H, LI C. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4[J]. Nat Commun,2013,4:1432−1439. doi: 10.1038/ncomms2401
|
| [7] |
ZHU J, FAN F, CHEN R, AN H, FENG Z, LI C. Direct imaging of highly anisotropic photogenerated charge separations on different facets of a single BiVO4 photocatalyst[J]. Angew Chem Int Ed Eng,2015,54:9111−9114. doi: 10.1002/anie.201504135
|
| [8] |
ZHU J, PANG S, DITTRICH T, GAO Y, NIE W, CUI J, CHEN R, AN H, FAN F, LI C. Visualizing the nano cocatalyst aligned electric fields on single photocatalyst particles[J]. Nano Lett,2017,17:6735−6741. doi: 10.1021/acs.nanolett.7b02799
|
| [9] |
ZHANG L, CHEN R, TU Y, GONG X, CAO X, XU Q, LI Y, YE B, YE Y, ZHU J. Revealing the crystal facet effect of ceria in Pd/CeO2 catalysts toward the selective oxidation of benzyl alcohol[J]. ACS Catal,2023,13:2202−2213. doi: 10.1021/acscatal.2c04252
|
| [10] |
MU L, ZHAO Y, LI A, WANG S, WANG Z, YANG J, WANG Y, LIU T, CHEN R, ZHU J, FAN F, LI R, LI C. Enhancing charge separation on high symmetry SrTiO3 exposed with anisotropic facets for photocatalytic water splitting[J]. Energy Environ. Sci.,2016,9:2463−2469. doi: 10.1039/C6EE00526H
|
| [11] |
TAN Y, ZHANG Z, GUO F, GUO R, BAI H, ZHANG B, LI X, YANG Q, LIU X. Effect of morphology transformation on photocatalytic performance of CdS crystal[J]. J Mater Sci: Mater Electron,2020,31:20315−20324. doi: 10.1007/s10854-020-04551-9
|
| [12] |
SONG S, QU J, HAN P, HULSEY M J, ZHANG G, WANG Y, WANG S, CHEN D, LU J, YAN N. Visible-light-driven amino acids production from biomass-based feedstocks over ultrathin CdS nanosheets[J]. Nat Commun,2020,11:4899−4909. doi: 10.1038/s41467-020-18532-3
|
| [13] |
CHAI Z, ZENG T T, LI Q, LU L Q, XIAO W J, XU D. Efficient visible light-driven splitting of alcohols into hydrogen and corresponding carbonyl compounds over a Ni-modified CdS photocatalyst[J]. J Am Chem Soc,2016,138:10128−10131. doi: 10.1021/jacs.6b06860
|
| [14] |
ZHANG L, JIANG D, IRFAN R M, TANG S, CHEN X, DU P. Highly efficient and selective photocatalytic dehydrogenation of benzyl alcohol for simultaneous hydrogen and benzaldehyde production over Ni-decorated Zn0.5Cd0.5S solid solution[J]. J Energy Chem,2019,30:71−77. doi: 10.1016/j.jechem.2018.03.014
|
| [15] |
ZHANG S, CHEN G, ZHU Z, WANG Y, WANG L, MENG S, ZHENG X, FU X, ZHANG F, HUANG W, CHEN S. Coordinating ultra-low content Au modified CdS with coupling selective oxidation and reduction system for improved photoexcited charge utilization[J]. J Catal,2021,402:72−82. doi: 10.1016/j.jcat.2021.08.028
|
| [16] |
LEE S G, KANG M J, PARK M, KIM K J, LEE H, KIM H S. Selective photocatalytic conversion of benzyl alcohol to benzaldehyde or deoxybenzoin over ion-exchanged CdS[J]. Appl Catal B: Environ,2022,304:120967−120979. doi: 10.1016/j.apcatb.2021.120967
|
| [17] |
ZHENG Z, WANG T, HAN F, YANG Q, LI B. Synthesis of Ni modified Au@CdS core-shell nanostructures for enhancing photocatalytic coproduction of hydrogen and benzaldehyde under visible light[J]. J Colloid Interface Sci,2022,606:47−56. doi: 10.1016/j.jcis.2021.07.150
|
| [18] |
SUN Z, ZHENG H, LI J, DU P. Extraordinarily efficient photocatalytic hydrogen evolution in water using semiconductor nanorods integrated with crystalline Ni2P cocatalysts[J]. Energy Environ Sci,2015,8:2668−2676. doi: 10.1039/C5EE01310K
|
| [19] |
JIANG D, SUN Z, JIA H, LU D, DU P. A cocatalyst-free CdS nanorod/ZnS nanoparticle composite for high-performance visible-light-driven hydrogen production from water[J]. J Mater Chem A,2016,4:675−683. doi: 10.1039/C5TA07420G
|
| [20] |
LI C, YUAN J, HAN B, SHANGGUAN W. Synthesis and photochemical performance of morphology-controlled CdS photocatalysts for hydrogen evolution under visible light[J]. Int J Hydrog Energy,2011,36:4271−4279. doi: 10.1016/j.ijhydene.2011.01.022
|
| [21] |
VAQUERO F, NAVARRO R M, FIERRO J L G. Influence of the solvent on the structure, morphology and performance for H2 evolution of CdS photocatalysts prepared by solvothermal method[J]. Appl Catal B: Environ,2017,203:753−767. doi: 10.1016/j.apcatb.2016.10.073
|
| [22] |
KONG X, YU F, ZHANG H, LV F, WANG Y, YIN L, HUANG J, FENG Q. Synthesis and study of morphology regulation, formation mechanism and photocatalytic performance of CdS[J]. Appl Surf Sci,2022,576:151817−151824. doi: 10.1016/j.apsusc.2021.151817
|
| [23] |
IQBAL S, PAN Z, ZHOU K. Enhanced photocatalytic hydrogen evolution from in situ formation of few-layered MoS2/CdS nanosheet-based van der Waals heterostructures[J]. Nanoscale,2017,9:6638−6642. doi: 10.1039/C7NR01705G
|
| [24] |
JANG J S, JOSHI U A, LEE J S. Solvothermal synthesis of CdS nanowires for photocatalytic hydrogen and electricity production[J]. J Phys Chem C,2007,111:13280−13287. doi: 10.1021/jp072683b
|
| [25] |
TAN Y, LIU X Y, LI L, KANG L, WANG A, ZHANG T. Effects of divalent metal ions of hydrotalcites on catalytic behavior of supported gold nanocatalysts for chemoselective hydrogenation of 3-nitrostyrene[J]. J Catal,2018,364:174−182. doi: 10.1016/j.jcat.2018.05.007
|
| [26] |
WANG Z, HISATOMI T, LI R, SAYAMA K, LIU G, DOMEN K, LI C, WANG L. Efficiency accreditation and testing protocols for particulate photocatalysts toward solar fuel production[J]. Joule,2021,5:344−359. doi: 10.1016/j.joule.2021.01.001
|
| [27] |
CHAI M Q, TAN Y, PEI G X, LI L, ZHANG L, LIU X Y, WANG A, ZHANG T. Crystal plane effect of ZnO on the catalytic activity of gold nanoparticles for the acetylene hydrogenation reaction[J]. J Phys Chem C,2017,121:19727−19734. doi: 10.1021/acs.jpcc.7b04022
|
| [28] |
CHEN J, FANG W, ZHANG Q, DENG W, WANG Y. A comparative study of size effects in the Au-catalyzed oxidative and non-oxidative dehydrogenation of benzyl alcohol[J]. Chem Asian J,2014,9:2187−2196. doi: 10.1002/asia.201402238
|
| [29] |
JIANG D, CHEN X, ZHANG Z, ZHANG L, WANG Y, SUN Z, IRFAN R M, DU P. Highly efficient simultaneous hydrogen evolution and benzaldehyde production using cadmium sulfide nanorods decorated with small cobalt nanoparticles under visible light[J]. J Catal,2018,357:147−153. doi: 10.1016/j.jcat.2017.10.019
|
| [30] |
MCCLELLAND K P, WEISS E A. Selective photocatalytic oxidation of benzyl alcohol to benzaldehyde or C–C coupled products by visible-light-absorbing quantum dots [J]. ACS Appl Energ Mater, 2018, 2: 92-96.
|
| [31] |
HAO H, ZHANG L, WANG W, QIAO S, LIU X. Photocatalytic hydrogen evolution coupled with efficient selective benzaldehyde production from benzyl alcohol aqueous solution over ZnS-NixSy composites[J]. ACS Sustainable Chem Eng,2019,7:10501−10508. doi: 10.1021/acssuschemeng.9b01017
|
| [32] |
ZHANG Z, YATES J T JR. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces[J]. Chem Rev,2012,112:5520−5551. doi: 10.1021/cr3000626
|
 2023-F004 Supporting Information.docx
2023-F004 Supporting Information.docx




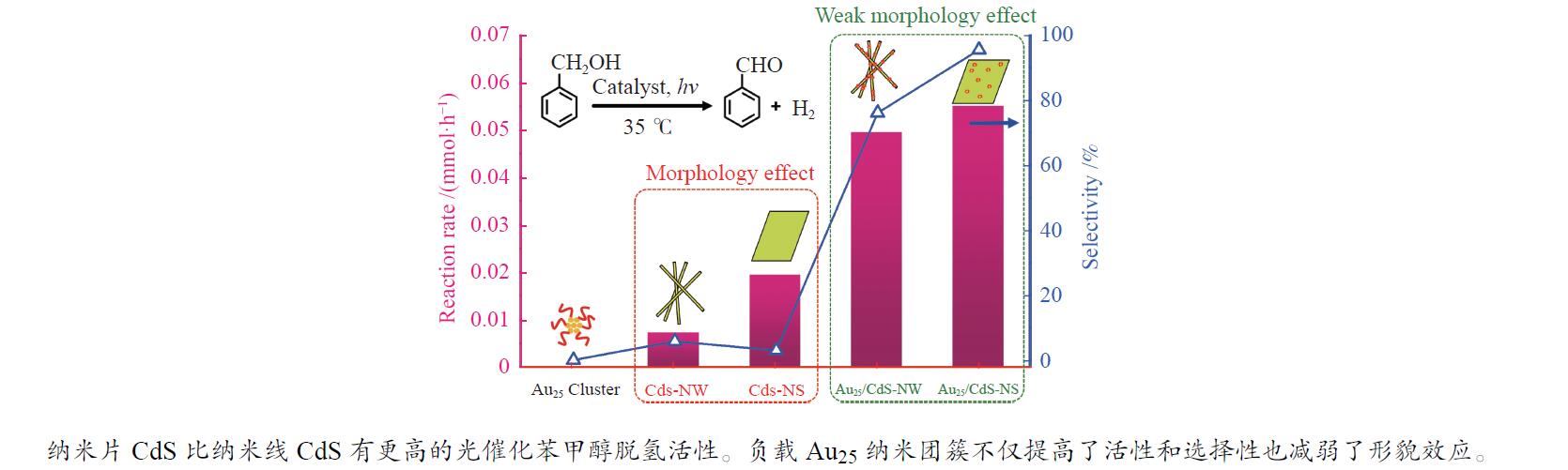
 下载:
下载: