Investigation on production of dimethyl carbonate from propylene carbonate and methanol on calcium cerium-based catalysts
-
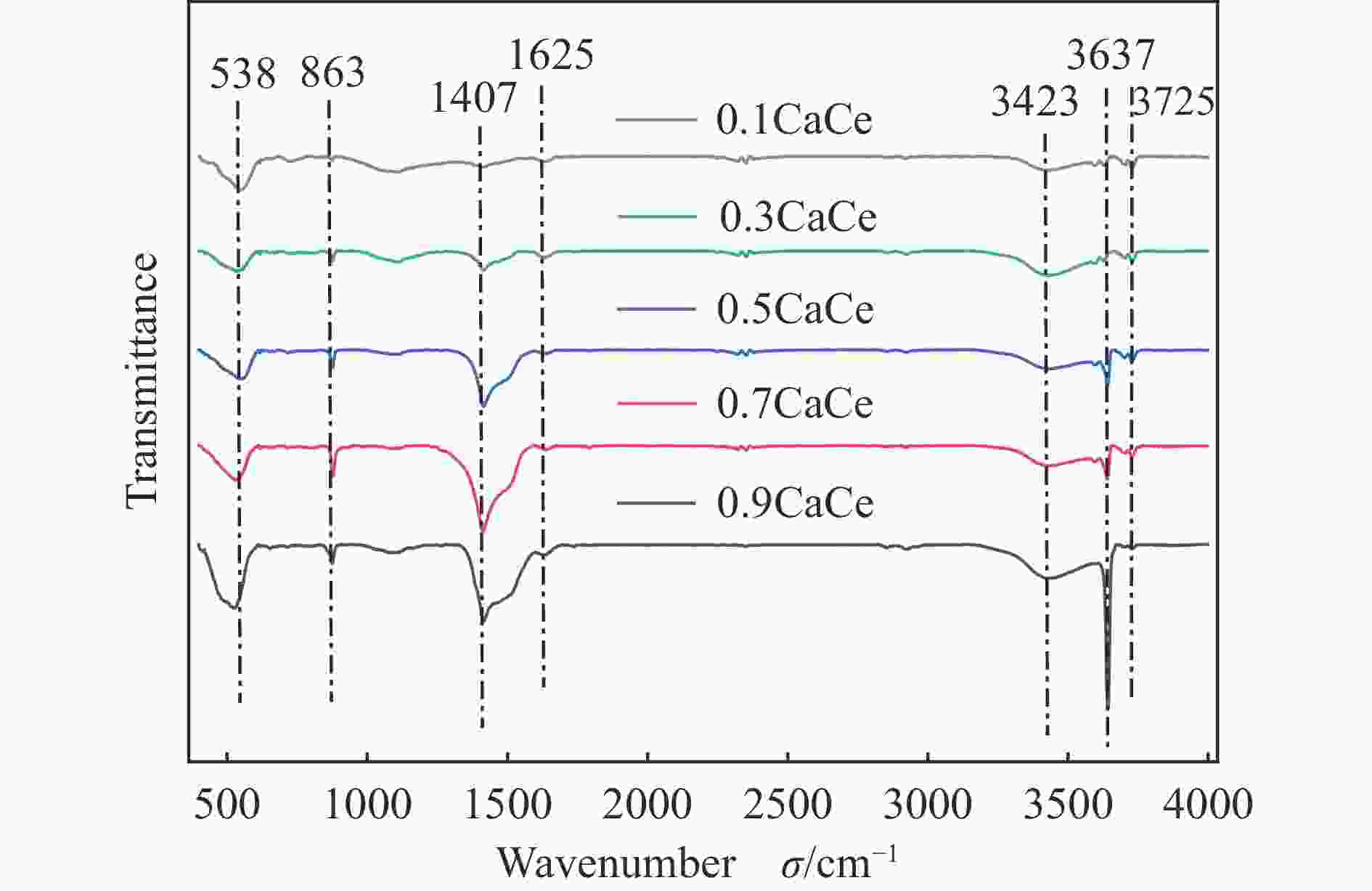
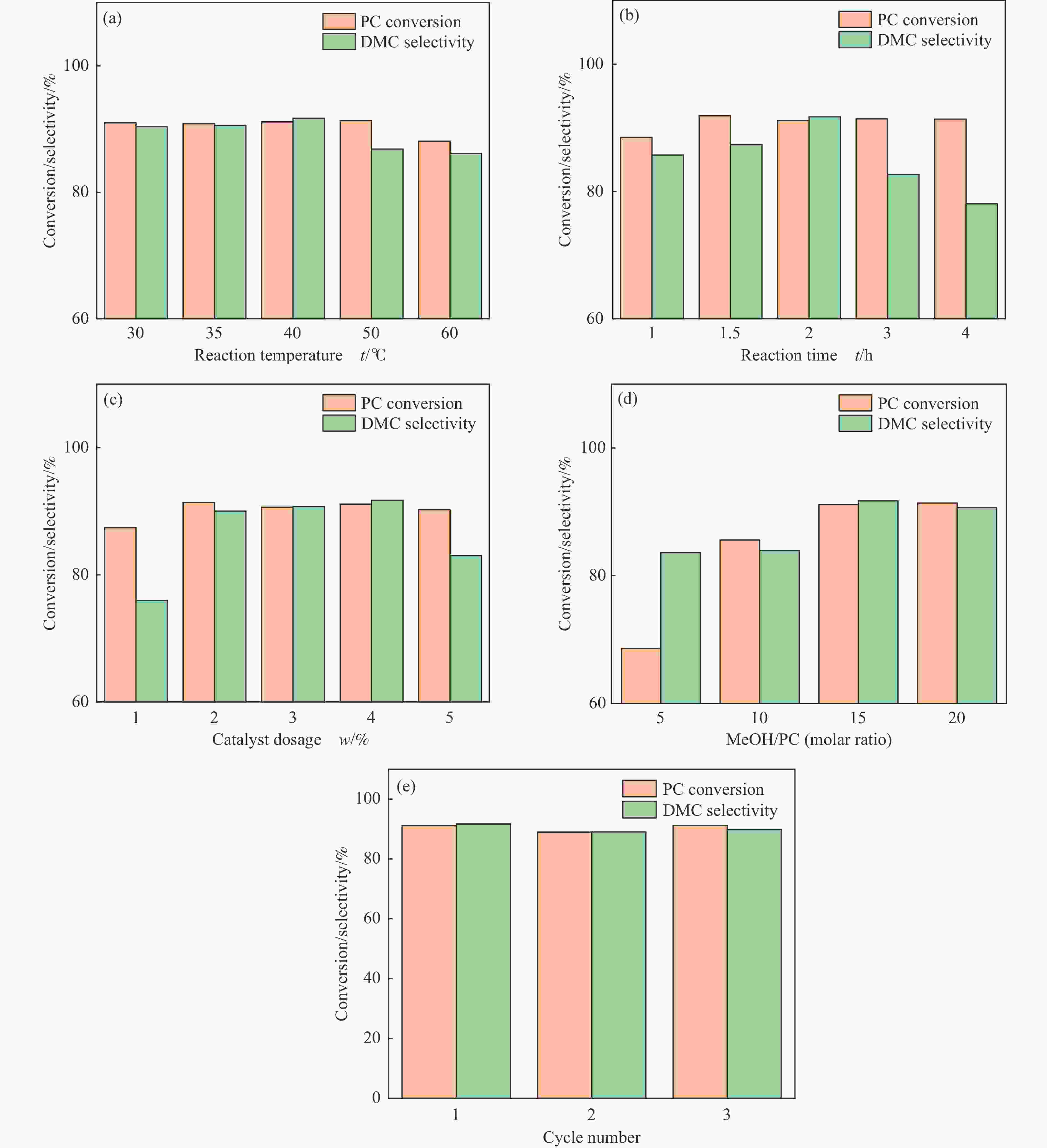
摘要: 采用溶胶凝胶法制备了不同比例的钙铈基催化剂,并研究了其对于碳酸丙烯酯和甲醇制备碳酸二甲酯的酯交换反应性能。结果表明, Ca∶Ce=9的催化剂在反应时间2 h,温度40 ℃,甲醇与碳酸丙烯酯物质的量比为15∶1,催化剂用量为碳酸丙烯酯用量4%的条件下,碳酸丙烯酯转化率达到91.1%,碳酸二甲酯选择性达到91.7%。采用XRD、N2吸附-脱附、FT-IR、XPS和CO2-TPD对催化剂进行了表征。结果表明,催化剂表面的氧空穴越多,中等碱性位数量越多,越有利于甲醇的活化,催化剂的活性越好。Abstract: Calcium cerium-based catalysts with different Ca:Ce molar ratio prepared by sol-gel method were characterized by XRD, N2 adsorption-desorption, FT-IR, XPS and CO2-TPD, and evaluated the activity for dimethyl carbonate (DMC) synthesis from propylene carbonate (PC) and methanol. The results indicated that more surface oxygen vacancies and more moderate basic sites are beneficial for methanol activation and thus leading to better catalytic activity. The PC conversion was 91.1% with DMC selectivity of 91.72% over 0.9CaCe under the reaction conditions-reaction time of 2 h, reaction temperature of 40 °C, methanol to propylene carbonate molar ratio of 15:1 and catalyst amount of 4% relative to the amount of PC.
-
Key words:
- calcium-cerium based catalysts /
- dimethyl carbonate /
- transesterification /
- solid base
-
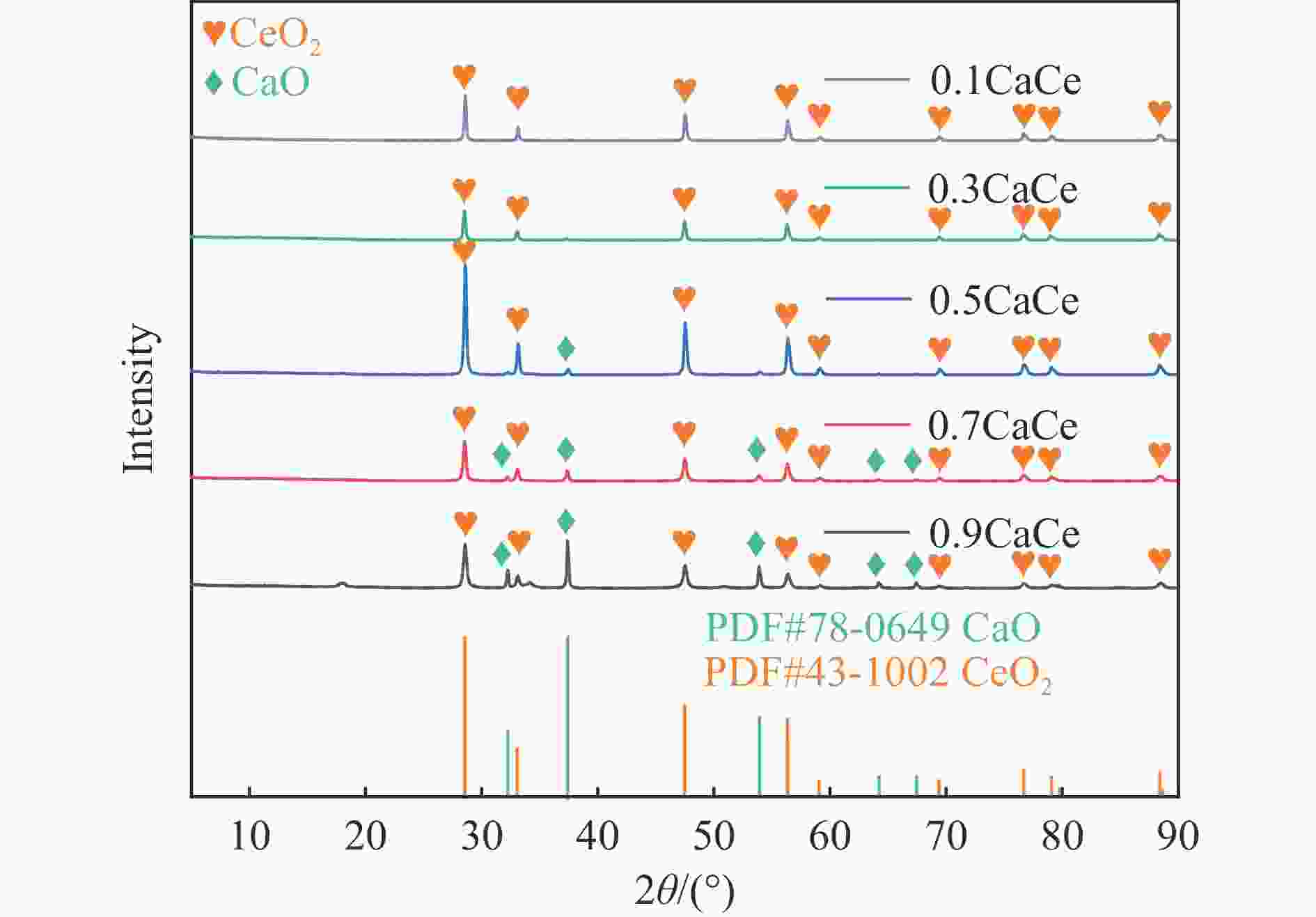
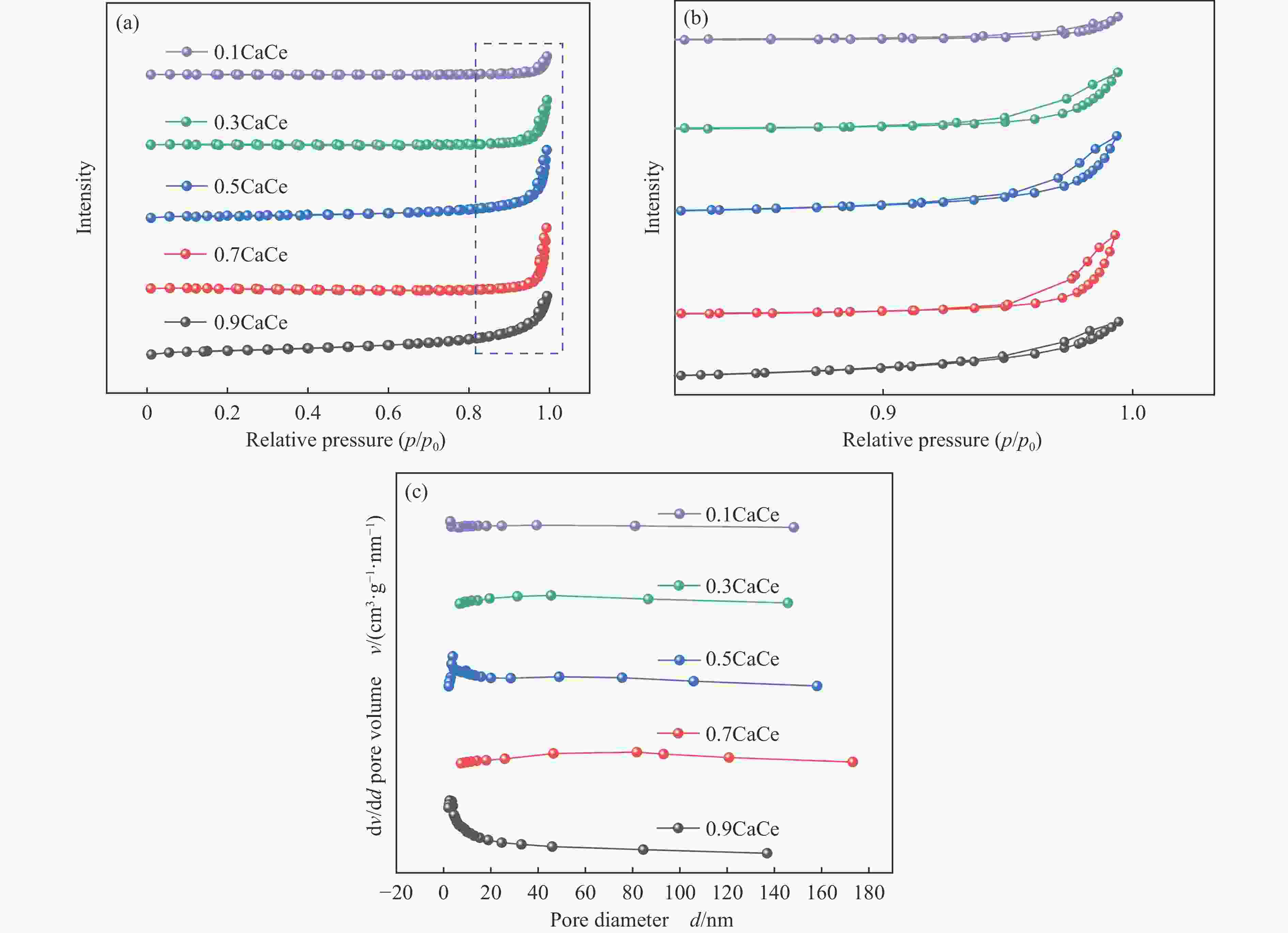
表 1 不同比例钙铈基催化剂的织构参数
Table 1 Textural parameters of the catalysts with different Ca∶Ce molar ratio
Catalyst SBET/
(m2·g−1)Pore volumea/
(cm3·g−1)Average pore
sizeb/nm0.9CaCe 10.9 0.03 13.0 0.7CaCe 1.8 0.02 60.1 0.5CaCe 5.9 0.02 26.9 0.3CaCe 1.6 0.01 48.7 0.1CaCe 0.7 0.01 39.4 a: Measured at p/p0=0.99; b: Calculated from isothermal desorption branches using the BJH method. 表 2 催化剂中Ca和O的结合能值
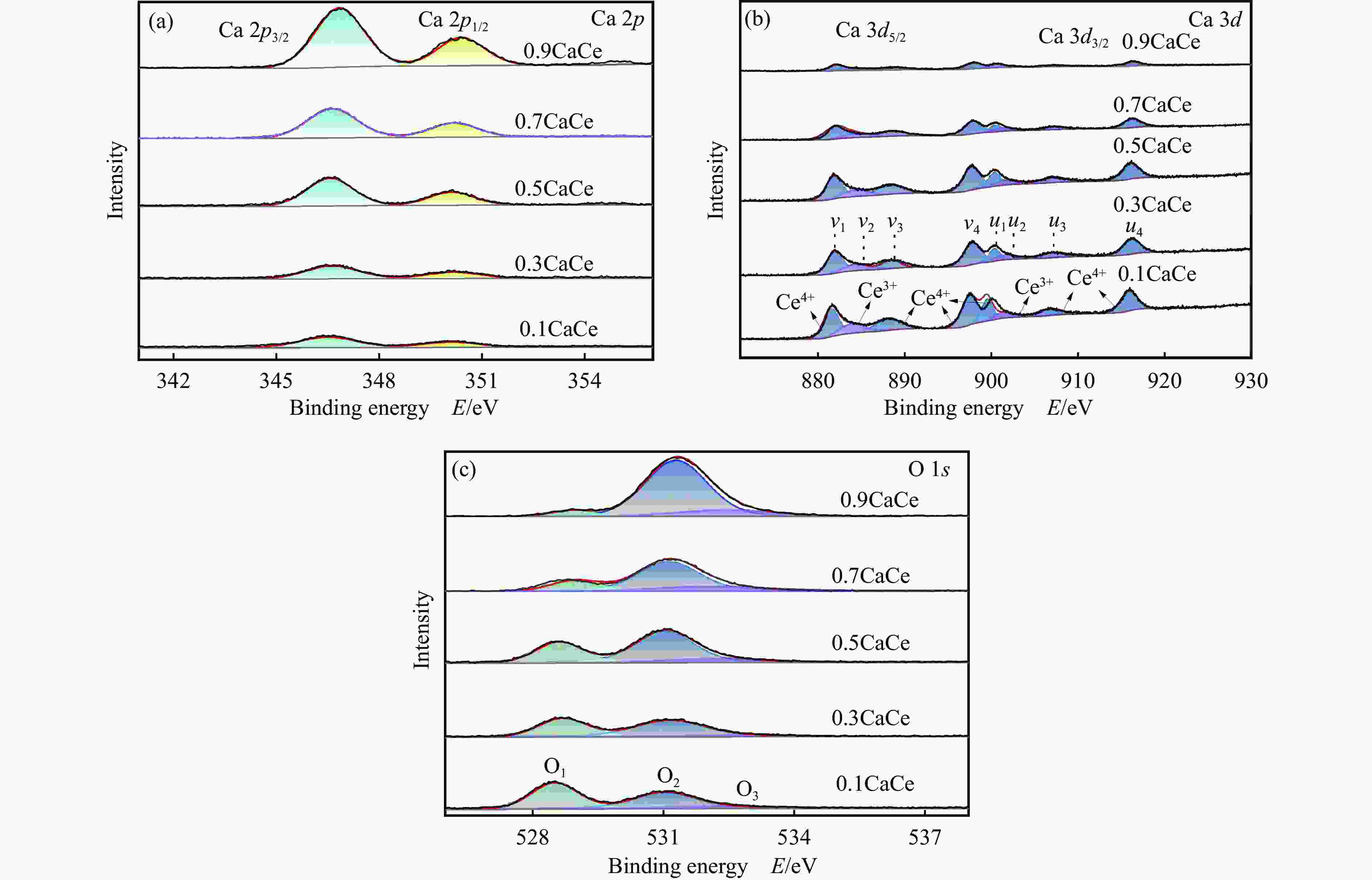
Table 2 Binding energy values of the catalysts
Catalyst Binding energy/eV Ca 2p O 1s 0.9CaCe 346.86, 350.41 528.89, 531.3, 532.42 0.7CaCe 346.64, 350.2 528.77, 531.13, 532.07 0.5CaCe 346.58, 350.13 528.61, 531.03, 532.25 0.3CaCe 346.64, 350.19 528.69, 531.14, 532.87 0.1CaCe 346.51, 350.1 528.51, 531.01, 532.42 表 3 催化剂表面氧物种的相对含量
Table 3 Relative content of oxygen species of the catalysts
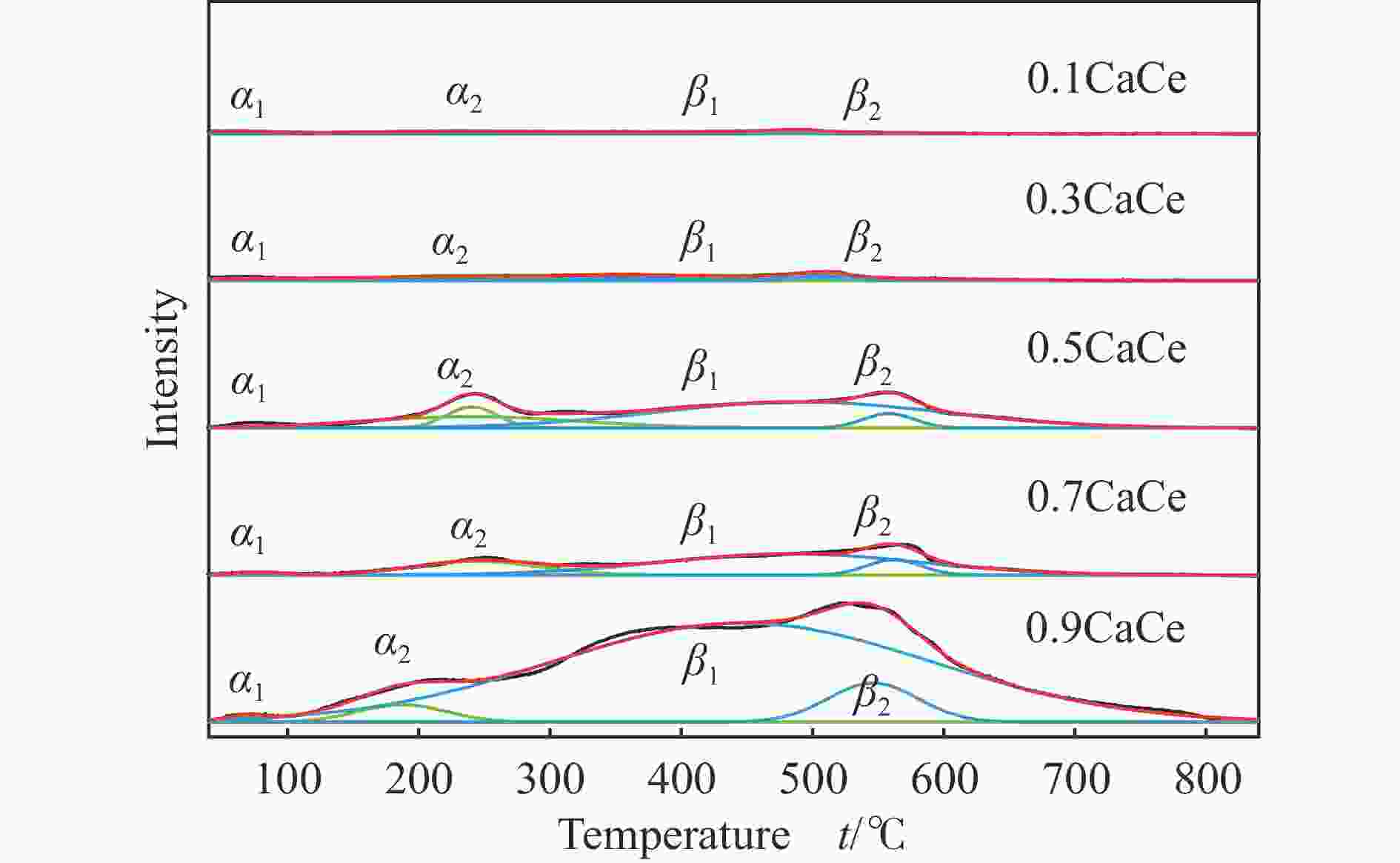
Catalyst O 1s/% O1 O2 O3 0.9CaCe 5.92 80.30 13.79 0.7CaCe 20.01 63.04 16.95 0.5CaCe 31.34 57.33 11.33 0.3CaCe 41.61 52.32 6.07 0.1CaCe 51.90 40.33 7.76 表 4 催化剂的总碱位数量和各碱性位点所占比例
Table 4 The total number and the proportion of each basic site of the catalysts
Catalyst α peak/% β peak/% Total basicity/(mmol·g−1) 0.9CaCe 3.96 96.04 1.85 0.7CaCe 22.76 77.24 0.35 0.5CaCe 23.98 76.02 0.52 0.3CaCe 44.81 55.19 0.12 0.1CaCe 53.31 46.69 0.04 表 5 钙铈基催化剂的反应性能评价
Table 5 Catalytic performance of the catalysts
Catalyst Reaction conditions PC
conv./%DMC
sel./%temp./℃ time/h CH3OH/PC mcat./mPC
/%0.1CaCe 60 2 15 4 32.0 6.3 0.3CaCe 60 2 15 4 61.3 45.7 0.5CaCe 60 2 15 4 74.3 87.5 0.7CaCe 60 2 15 4 88.9 87.4 0.9CaCe 60 2 15 4 89.4 87.4 CeO2 60 2 15 4 43.7 0.0 -
[1] AHMAD RUSLAN N A A, KAN S Y, HAMZAH A S, et al. Natural food additives as green catalysts in organic synthesis: A review[J]. Environ Chem Lett,2021,19(4):3359−3380. doi: 10.1007/s10311-021-01209-8 [2] SHARMA S, DAS J, BRAJE W M, et al. A glimpse into green chemistry practices in the pharmaceutical industry[J]. ChemSusChem,2020,13(11):2859−2875. doi: 10.1002/cssc.202000317 [3] TUNDO P, MUSOLINO M, ARICO F. The reactions of dimethyl carbonate and its derivatives[J]. Green Chem,2018,20(1):28−85. doi: 10.1039/C7GC01764B [4] DABRAL S, ENGEL J, MOTTWEILER J, et al. Mechanistic studies of base-catalysed lignin depolymerisation in dimethyl carbonate[J]. Green Chem,2018,20(1):170−182. doi: 10.1039/C7GC03110F [5] GAO Y, LI Z, SU K, et al. Excellent performance of TiO2(B) nanotubes in selective transesterification of DMC with phenol derivatives[J]. Chem Eng J,2016,301:12−18. doi: 10.1016/j.cej.2016.04.036 [6] DU Z, ZHOU B, XIONG J, et al. Advances in catalyst for synthesis of dimethyl carbonate by oxidative carbonylation of methanol[J]. Chem Eng,2012,40(8):29−32. [7] ZHAO Y, LIU S, WANG G, et al. Progress in synthesis of dimethyl carbonate from urea[J]. Chem Ind Eng Prog,2004,23(10):1049−1052. [8] YANHONG C, HUAJUN W. Progress in synthesis of dimethyl carbonate via transesterification[J]. Chem Ind Eng Prog,2007,26(5):642−646. [9] LI H S, ZHONG S H. Dimethyl carbonate synthesis from carbon dioxide and methanol[J]. Prog Chem,2002,14(5):368−373. [10] KOHLI K, SHARMA B K, PANCHAL C B. Dimethyl carbonate: Review of synthesis routes and catalysts used[J]. Energies,2022,15(14):5133. [11] HOLTBRUEGGE J, KUHLMANN H, LUTZE P. Process analysis and economic optimization of intensified process alternatives for simultaneous industrial scale production of dimethyl carbonate and propylene glycol[J]. Chem Eng Res Des,2015,93:411−431. [12] AN H, ZHANG G, ZHAO X, et al. Preparation of highly stable Ca-Zn-Al oxide catalyst and its catalytic performance for one-pot synthesis of dimethyl carbonate[J]. Catal Today,2018,316:185−192. doi: 10.1016/j.cattod.2018.03.006 [13] LI F, LIAO Y H, ZHAO N, et al. The effect of NaF amount on solid base catalysts derived from F-Ca-Mg-Al layered double hydroxides and dimethyl carbonate synthesis[J]. J Fuel Chem Technol,2022,50(1):80−89. doi: 10.1016/S1872-5813(21)60165-2 [14] AHIRE J, BHANAGE B M. Solar light assisted synthesis of CeO2 nanoparticles for transesterification of ethylene carbonate with methanol to dimethyl carbonate[J]. Catal Lett,2022,152(11):3284−3293. doi: 10.1007/s10562-022-03927-2 [15] SHI Y B, ZHANG G L, SUN Y C, et al. KIT-6 supported CeO2 for catalytic synthesis of dimethyl carbonate from CO2 and methanol[J]. Chin J Inorg Chem,2021,37(6):1004−1016. [16] KUMAR N, SRIVASTAVA V C. Dimethyl carbonate synthesis via transesterification of propylene carbonate using an efficient reduced graphene oxide-supported ZnO nanocatalyst[J]. Energy Fuels,2020,34(6):7455−7464. [17] TIAN X, ZENG Y, XIAO T, et al. Fabrication and stabilization of nanocrystalline ordered mesoporous MgO-ZrO2 solid solution[J]. Microporous Mesoporous Mater,2011,143(2/3):357−361. doi: 10.1016/j.micromeso.2011.03.015 [18] XU J, CHEN Y, MA D, et al. Simple preparation of MgO/g-C3N4 catalyst and its application for catalytic synthesis of dimethyl carbonate via transesterification[J]. Catal Commun,2017,95:72−76. doi: 10.1016/j.catcom.2017.03.009 [19] WANG H, WANG M, ZHANG W, et al. Synthesis of dimethyl carbonate from propylene carbonate and methanol using CaO-ZrO2 solid solutions as highly stable catalysts[J]. Catal Today,2006,115(1/4):107−110. doi: 10.1016/j.cattod.2006.02.031 [20] WEI T, WANG M H, WEI W, et al. Effect of base strength and basicity on catalytic behavior of solid bases for synthesis of dimethyl carbonate from propylene carbonate and methanol[J]. Fuel Process Technol,2003,83(1/3):175−182. doi: 10.1016/S0378-3820(03)00065-1 [21] YOU Q, YIN X, WANG J, et al. A recyclable solid catalyst of KF/Ca-Mg-Al-O using for biodiesel production from jatropha seed oil: Preparation, characterization, and methanolysis process optimization[J]. Mater Res Exp,2022,9(6):065505. [22] CAKIRCA E E, AKIN A N. Study on heterogeneous catalysts from calcined Ca riched hydrotalcite like compounds for biodiesel production[J]. Sustainable Chem Pharm,2021,20:100378. [23] KUMAR P, SRIVASTAVA V C, MISHRA I M. Synthesis and characterization of Ce-La oxides for the formation of dimethyl carbonate by transesterification of propylene carbonate[J]. Catal Commun,2015,60:27−31. doi: 10.1016/j.catcom.2014.11.006 [24] LUO J, WANG Y, WANG F, et al. Aerobic oxidation of fluorene to fluorenone over copper-doped Co3O4 with a high specific surface area[J]. ACS Sustainable Chem Eng,2020,8(6):2568−2576. [25] THOMMES M, KANEKO K, NEIMARK A V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure Appl Chem,2015,87(9/10):1051−1069. doi: 10.1515/pac-2014-1117 [26] WU X, KANG M, ZHAO N, et al. Dimethyl carbonate synthesis over ZnO-CaO bi-functional catalysts[J]. Catal Commun,2014,46:46−50. doi: 10.1016/j.catcom.2013.10.040 [27] JI X, YANG J, ZHAO N, et al. Synthesis of ethylene carbonate by alcoholysis of urea over Zn-Zr mixed oxides[J]. Inorg Chem Commun,2021,134:109061. [28] LI F, WANG Y F, YANG Q Z, et al. Study on adsorption of glyphosate (N-phosphonomethyl glycine) pesticide on MgAl-layered double hydroxides in aqueous solution[J]. J Hazardous Mater,2005,125(1/3):89−95. doi: 10.1016/j.jhazmat.2005.04.037 [29] LIAO Y, LI F, PU Y, et al. Solid base catalysts derived from Ca-Al-X (X=F−, Cl− and Br−) layered double hydroxides for methanolysis of propylene carbonate[J]. Rsc Adv,2018,8(2):785−791. doi: 10.1039/C7RA10832J [30] PAL D B, LAVANIA R, SRIVASTAVA P, et al. Photo-catalytic degradation of methyl tertiary butyl ether from wastewater using CuO/CeO2 composite nanofiber catalyst[J]. J Environ Chem Eng,2018,6(2):2577−2587. doi: 10.1016/j.jece.2018.04.001 [31] AL-DARWISH J, SENTER M, LAWSON S, et al. Ceria nanostructured catalysts for conversion of methanol and carbon dioxide to carbonate[J]. Catal Today,2020,350:120−126. doi: 10.1016/j.cattod.2019.06.013 [32] HUO L, WANG T, PU Y, et al. Effect of cobalt doping on the stability of CaO-based catalysts for dimethyl carbonate Synthesis via the transesterification of propylene carbonate with methanol[J]. Chemistryselect,2021,6(38):10226−10237. doi: 10.1002/slct.202102987 [33] 刘春宇, 宋忠贤, 张学军, 等. 过渡金属改性Ce-M-Ox(M=Cu, Co, Mn和Fe)催化剂在NH3-SCR反应研究[J]. 化学试剂,2023,45(3):53−60.LIU Chunyu, SONG Zhongxian, ZHANG Xuejun, et al. Study on transition-metal-modified Ce-M-Ox(M=Cu, Co, Mn, and Fe) catalysts in the NH3-SCR reaction[J]. Chem Reag,2023,45(3):53−60. [34] WANG J, YANG L, LUO W, et al. Sustainable biodiesel production via transesterification by using recyclable Ca2MgSi2O7 catalyst[J]. Fuel,2017,196:306−313. doi: 10.1016/j.fuel.2017.02.007 [35] MARCINIUK L L, HAMMER P, PASTORE H O, et al. Sodium titanate as basic catalyst in transesterification reactions[J]. Fuel,2014,118:48−54. doi: 10.1016/j.fuel.2013.10.036 [36] LIU B, LI C, ZHANG G, et al. Oxygen vacancy promoting dimethyl carbonate synthesis from CO2 and methanol over Zr-doped CeO2 nanorods[J]. ACS Catal,2018,8(11):10446−10456. doi: 10.1021/acscatal.8b00415 [37] 王兰心, 于雪莲, 安晓强. 基于氧空位构筑路易斯酸碱位点及其在环境光催化中的应用[J]. 南京信息工程大学学报(自然科学版),2023,15(3):253−266.Wang Lanxin, YU Xuelian, AN Xiaoqiang. Constructing lewis acid-base sites based on oxygen vacancies and their application in environmental photocatalysis[J]. J Nanjing Univ Inform Sci Technol (Nat Sci Ed),2023,15(3):253−266. [38] CUTRUFELLO M G, ATZORI L, MELONI D, et al. Synthesis of dimethyl carbonate by transesterification of propylene carbonate with methanol on CeO2-La2O3 oxides prepared by the soft template method[J]. Materials,2021,14(17):4802. [39] FU Z, ZHONG Y, YU Y, et al. TiO2-doped CeO2 nanorod catalyst for direct conversion of CO2 and CH3OH to dimethyl carbonate: Catalytic performance and kinetic study[J]. ACS Omega,2018,3(1):198−207. doi: 10.1021/acsomega.7b01475 [40] KUMAR P, KAUR R, VERMA S, et al. The preparation and efficacy of SrO/CeO2 catalysts for the production of dimethyl carbonate by transesterification of ethylene carbonate[J]. Fuel,2018,220:706−716. doi: 10.1016/j.fuel.2018.01.137 [41] SMOLAKOVA L, FROLICH K, TROPPOVA I, et al. Determination of basic sites in Mg-Al mixed oxides by combination of TPD-CO2 and CO2 adsorption calorimetry[J]. J Therm Anal Calorim,2017,127(3):1921−1929. doi: 10.1007/s10973-016-5851-6 -





 下载:
下载: