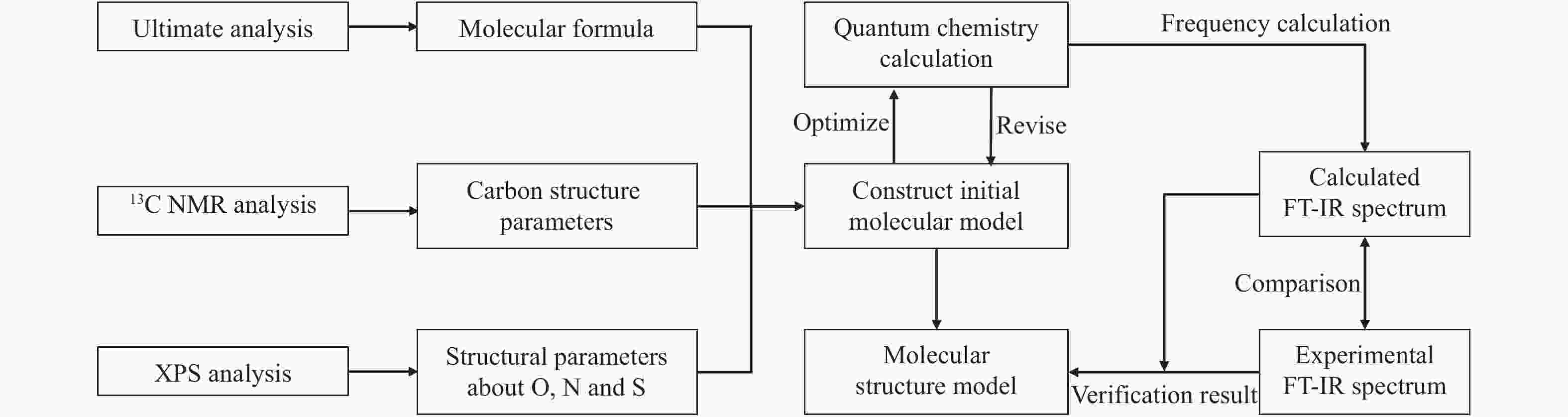
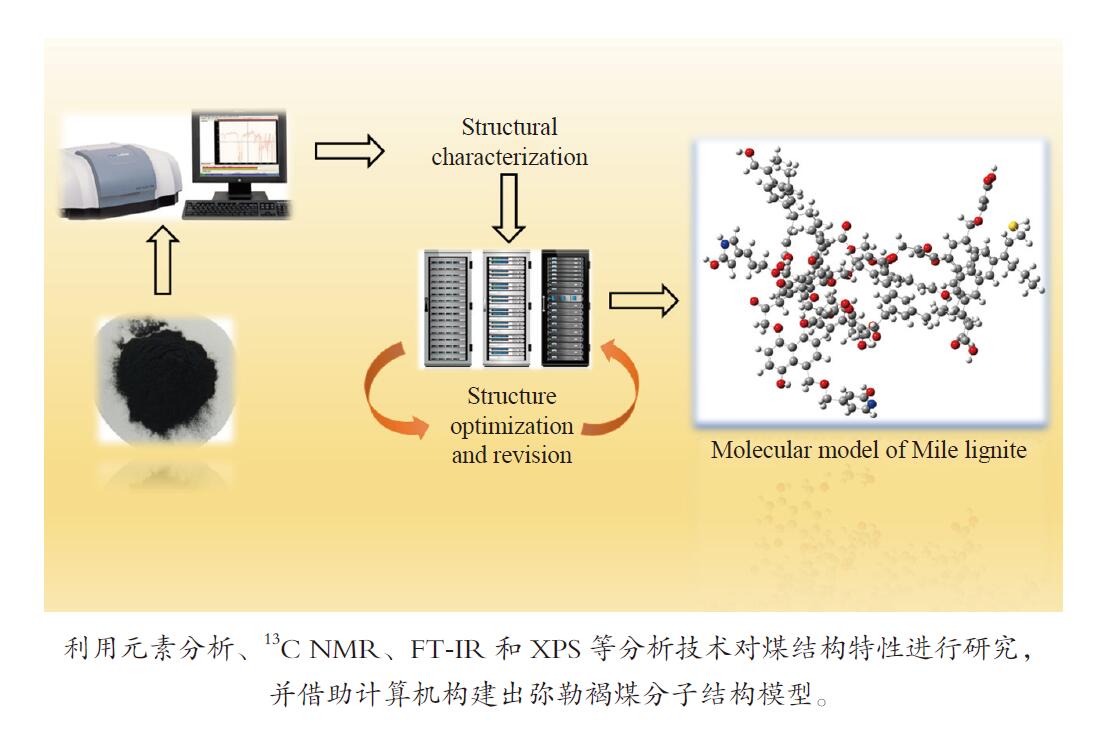
-
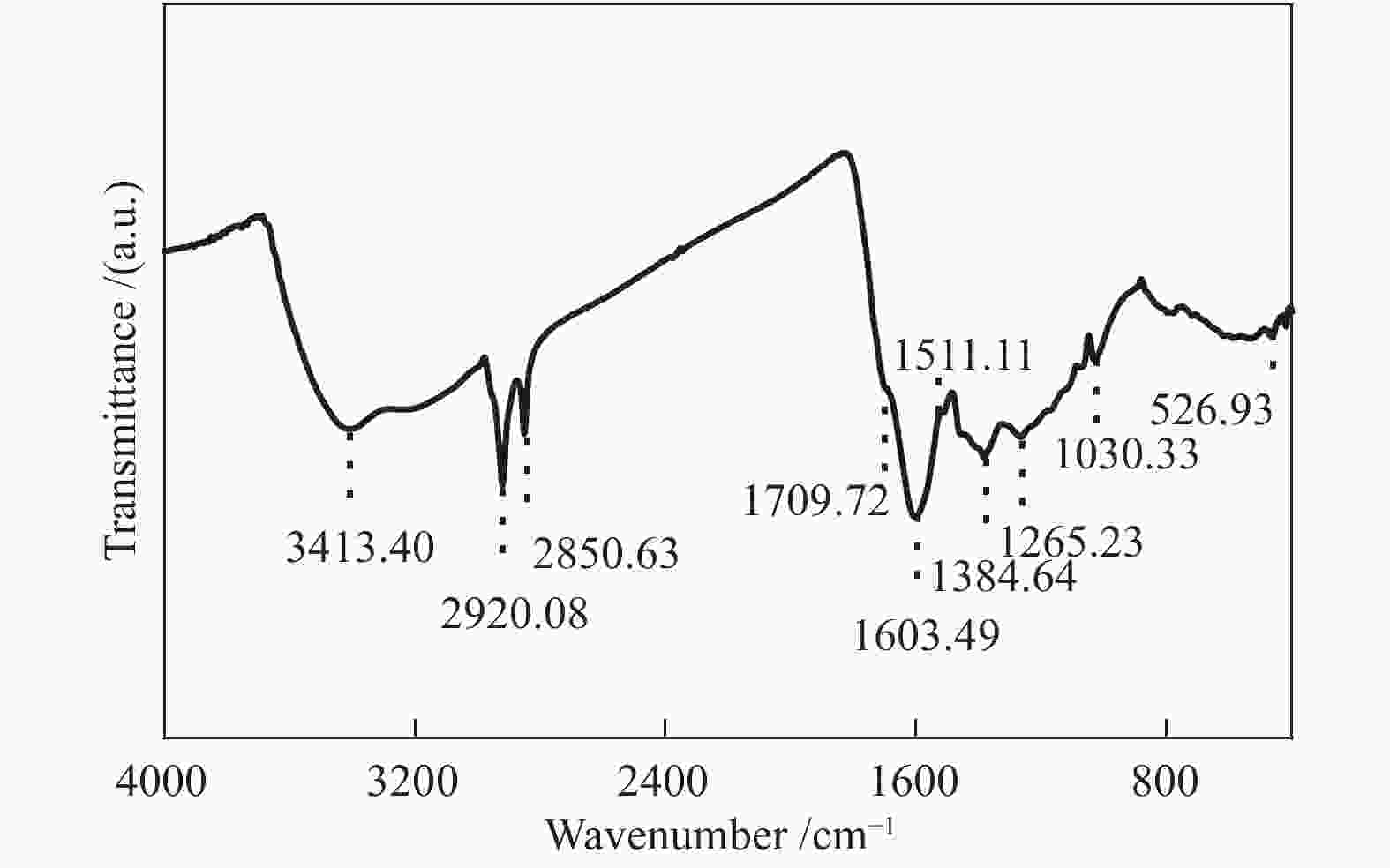
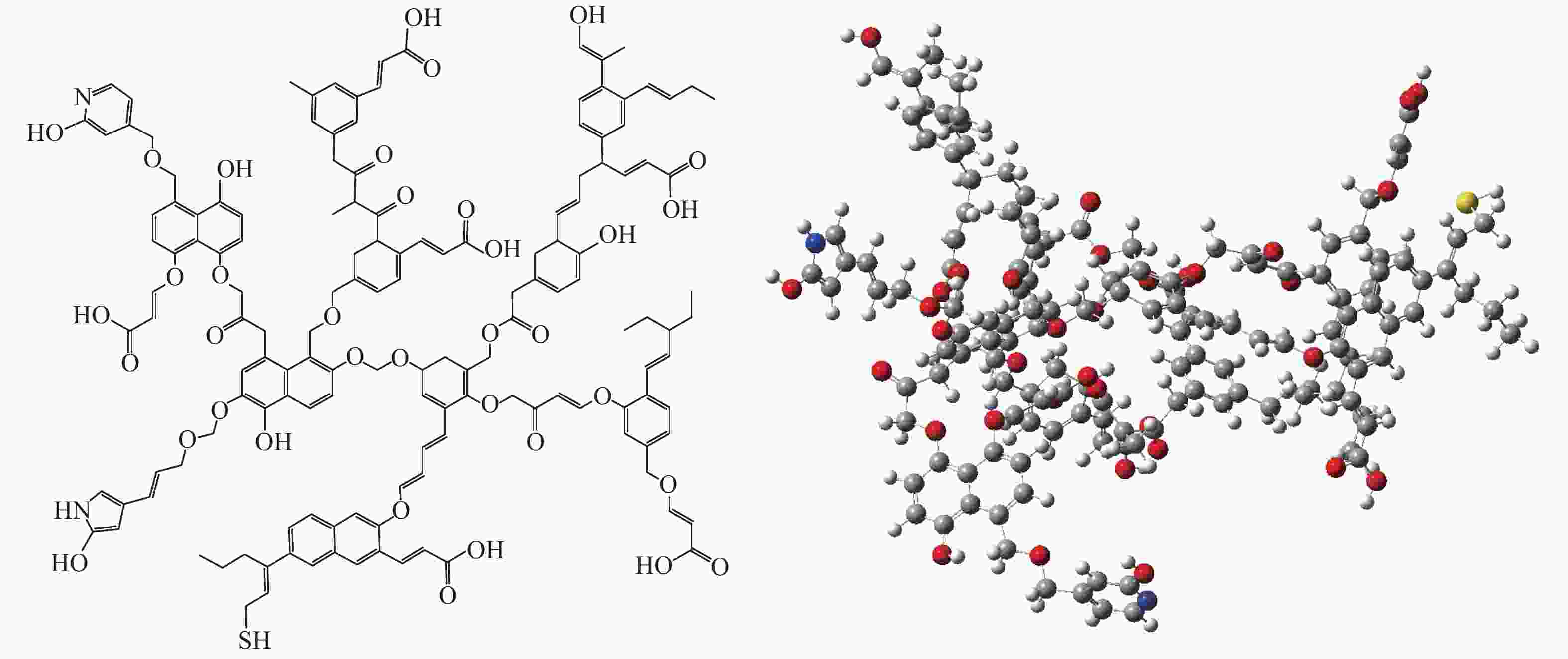
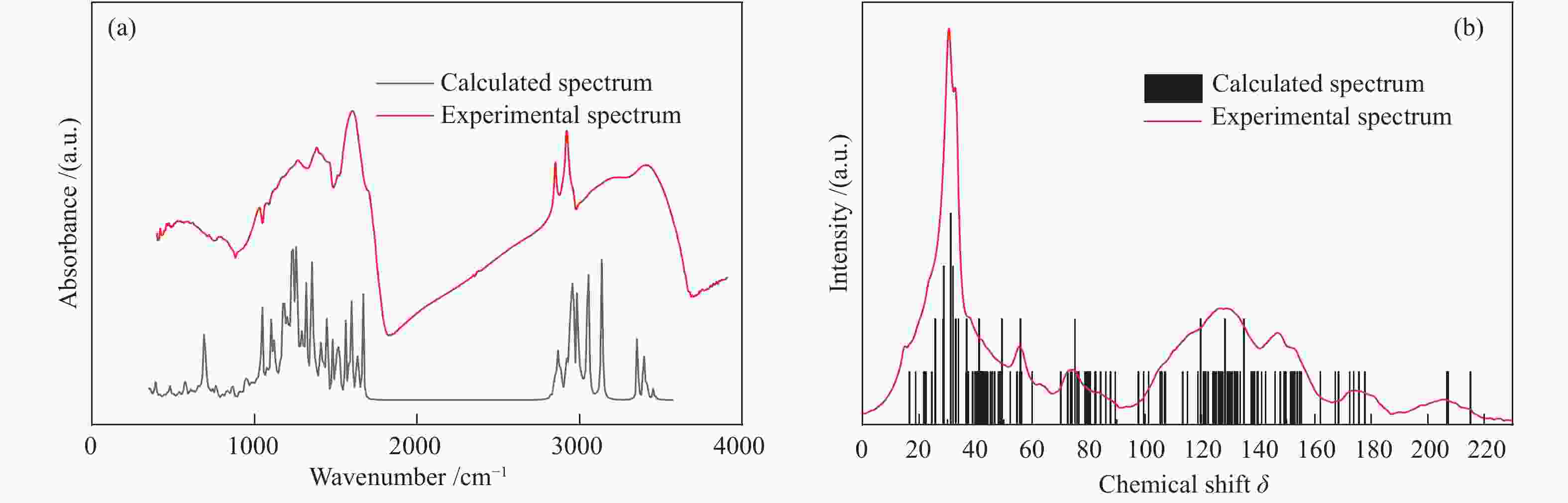
摘要: 以云南弥勒褐煤为研究对象,采用元素分析、傅里叶变换红外光谱、13C固体核磁共振波谱以及X射线光电子能谱等现代分析技术,获取了弥勒褐煤的结构参数信息。其中,芳香度为38.79%,芳香环取代基数量为3。芳香碳结构主要为苯和萘,脂肪碳结构以甲基、亚甲基为主;氧主要存在于醚氧、羧基和羰基中;氮主要以吡咯氮和吡啶氮的形式存在;硫主要为硫酚或硫醇。根据分析结果,构建出弥勒褐煤的分子结构模型,分子式为C147H148O36N2S。采用半经验法PM3基组和密度泛函理论M06-2X泛函对分子构型进行优化,优化后的模型立体构型显著,且芳香层片在空间上呈现不规则排列,各芳香环之间主要通过甲基、亚甲基、甲氧基以及脂肪环连接。模拟FT-IR光谱和模拟 13C NMR波谱均与实验谱图吻合良好,证明了弥勒褐煤分子模型的准确性和合理性。Abstract: The understanding of the structural characteristics of lignite is of very importance to the lignite utilization. The structure parameters of Mile lignite in Yunnan were ascertained via Fourier transform infrared spectroscopy, 13C solid-state nuclear magnetic resonance spectroscopy, X-ray photoelectron spectroscopy and ultimate analysis. The results indicate that the aromaticity and number of aromatic ring substituents of Mile lignite are 38.79% and 3, respectively. The aromatic carbon structure typically contains benzene and naphthalene, while the aliphatic carbon structure mainly includes methyl and methylene. Oxygen dominantly exists in ether oxygen, carboxyl and carbonyl; nitrogen occurs in the form of pyrrole nitrogen and pyridine nitrogen; and sulfur is mainly present in thiophenol and mercaptan. According to the analysis results, a molecular structure model of Mile lignite is constructed, with a molecular formula of C147H148O36N2S. The semi-empirical PM3 basis set and the density functional theory M06-2X were adopted to optimize the molecular configuration. The optimized model has a significant three-dimensional configuration, in which the aromatic layers are arranged irregularly in space and the aromatic rings are connected by methyl, methylene, methoxy and alicyclic rings. The simulated FT-IR spectrum and simulated 13C NMR spectrum are in great agreement with the experimental spectrum, which proves the accuracy and rationality of the molecular model of Mile lignite.
-
表 1 ML的工业分析与元素分析
Table 1 Proximate analysis and Ultimate analysis of ML
Proximate analysis w/% Ultimate analysis wdaf/% Mad Ad Vdaf FCdaf C H N St O 7.22 9.55 59.18 40.82 69.66 5.83 1.10 0.83 22.58 notes: Mad is the moisture mass fraction of sample on the air dry basis; Ad is the ash mass fraction of sample on the dry basis; Vdaf is the volatile matter mass fraction of sample on the dry ash-free basis; FCdaf is the fixed carbon mass fraction of sample on the dry ash-free basis; St is the total sulfur 表 2 ML 13C NMR分峰获得的含碳官能团的化学位移和含量
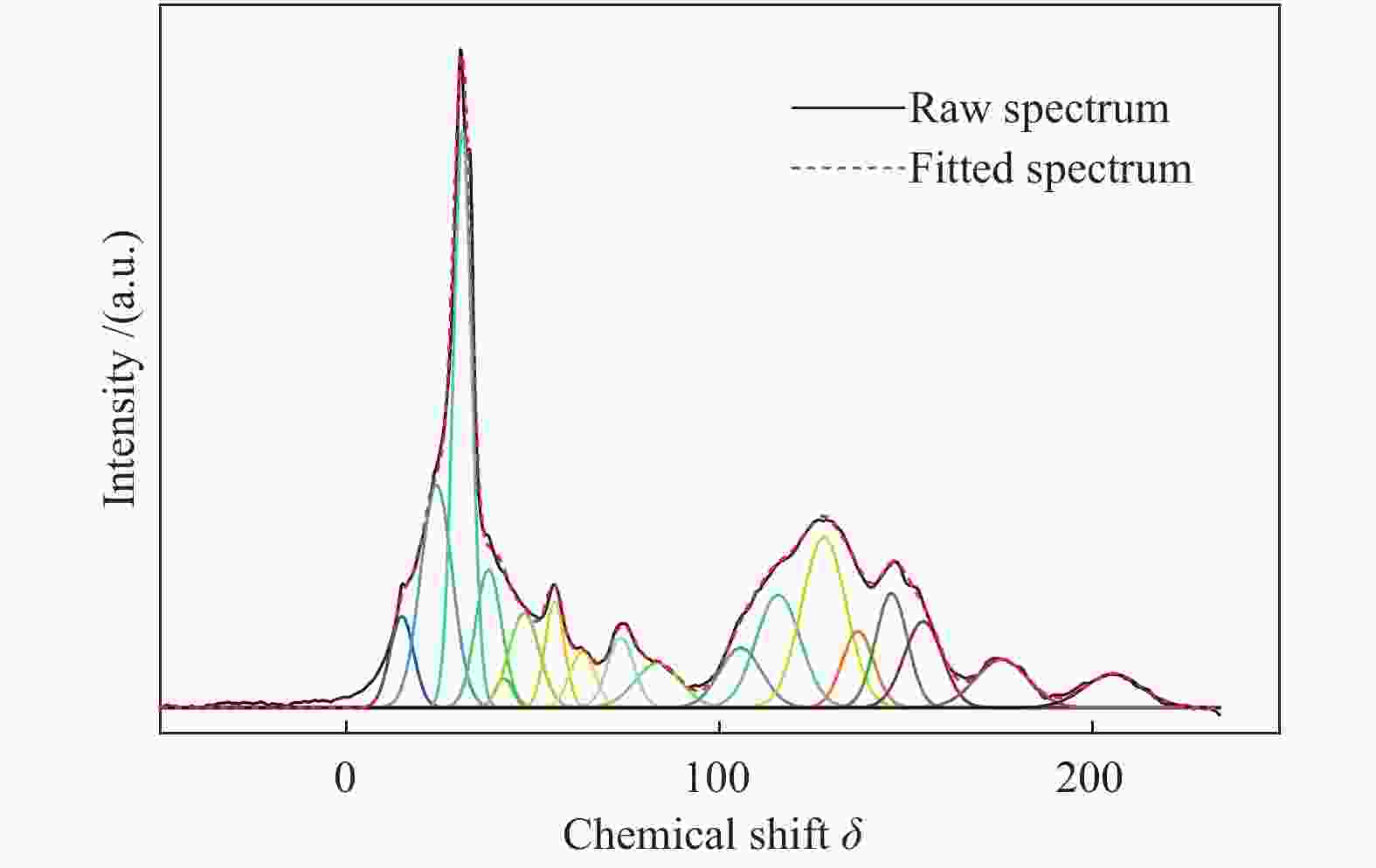
Table 2 The chemical shifts and the contents of carbon-containing functional groups obtained from 13C NMR peaks of ML
Carbon type Chemical
shift δSymbol Molar content/% Aliphatic aliphatic CH3 15 fal1 3.40 aromatic CH3 20 fala 11.20 methylene 25, 31 fal2 22.60 quaternary sp3C 40, 47 fal3 5.36 oxygen aliphatic carbon 55, 63, 74, 83 falo 11.62 Aromatic aromatic protonated 105, 115, 127 faH 23.95 aromatic bridgehead 137 fab 3.84 alkylated aromatic 146 fas 5.80 oxygen aromatic carbon 154 fao2 5.20 Carbonyl carboxyl carbon 173 faC1 4.00 carbonyl carbon 205 faC2 3.03 表 3 ML的XPS分析
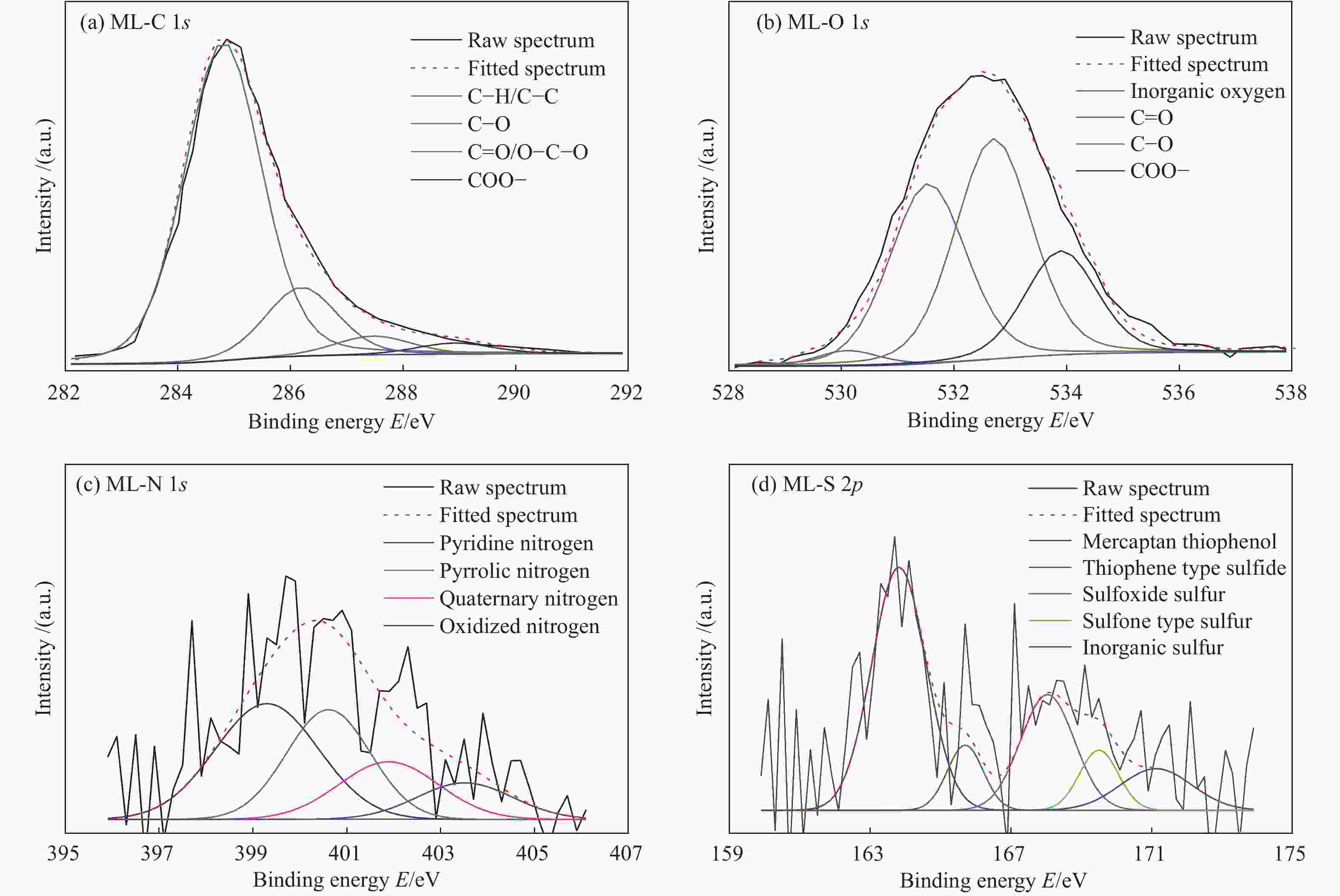
Table 3 XPS analysis results of ML
Functional group Binding energy E/eV Content/% C 1s C−C/C−H 284.8(± 0.2) 77.22 C−O 286.3(± 0.2) 15.61 C=O/O−C−O 287.5(± 0.2) 4.51 COOH 289.0(± 0.2) 2.66 O 1s inorganic oxygen 530.3(± 0.2) 2.45 C=O/O−C−O 531.4(± 0.2) 35.24 C−O 532.5(± 0.2) 43.20 COOH 533.9(± 0.2) 19.10 N 1s pyridine nitrogen 399.5(± 0.2) 39.45 pyrrolic nitrogen 400.5(± 0.2) 30.22 quaternary nitrogen 401.3(± 0.2) 18.89 oxidized nitrogen 402.8(± 0.2) 11.44 S 2p mercaptan thiophenol 163.8(± 0.2) 48.82 thiophene type sulfide 165.7(± 0.2) 8.62 sulfoxide sulfur 168.0(± 0.2) 22.97 sulfone type sulfur 169.5(± 0.2) 8.62 inorganic sulfur 171.1(± 0.2) 10.97 表 4 ML分子结构中芳香结构的种类和数量
Table 4 Types and quantities of aromatic structures in ML molecules
Type Aromatic unit structure Number Benzene 
3 Naphthalene 
3 Pyridine 
1 Pyrrole 
1 -
[1] 童国通, 吴荣生. 宝日希勒褐煤在合成气与复合溶剂系统下的液化性能研究[J]. 燃料化学学报,2019,47(6):661−667. doi: 10.3969/j.issn.0253-2409.2019.06.003TONG Guo-tong, WU Rong-sheng. Liquefaction characteristics of Baorixile lignite with syngas and complex solvent[J]. J Fuel Chem Technol,2019,47(6):661−667. doi: 10.3969/j.issn.0253-2409.2019.06.003 [2] FENG L, ZHAO G Y, ZHAO Y Y, ZHAO M S, TANG J W. Construction of the molecular structure model of the Shengli lignite using TG-GC/MS and FTIR spectrometry data[J]. Fuel,2017,203:924−931. doi: 10.1016/j.fuel.2017.04.112 [3] CONG X S, ZONG Z M, WEI Z H. Enrichment and identification of arylhopanes from Shengli lignite[J]. Energy Fuels,2014,28:6745−6748. doi: 10.1021/ef501105n [4] CONG X S, ZONG Z M, ZHOU Y, LI M, WANG W L, LI F G, ZHOU J, FAN X, ZHANG Y P, WEI X Y. Isolation and identification of 3-ethyl-8-methyl-2, 3-dihydro-1H-cyclopenta[a]chrysene from Shengli lignite[J]. Energy Fuels,2014,28(10):6694−6697. doi: 10.1021/ef402403y [5] MOCHIDA I, OKUMA O, YOON S H. Chemicals from direct coal liquefaction[J]. Chem Rev,2014,114(3):1637−72. doi: 10.1021/cr4002885 [6] 冯炜, 高红凤, 王贵, 吴浪浪, 许靖钦, 李壮楣, 李平, 白红存, 郭庆杰. 枣泉煤分子模型构建及热解的分子模拟[J]. 化工学报,2019,70(4):1522−1531.FENG Wei, GAO Hong-feng, WANG Gui, XU Jing-qin, LI Zhuang-mei, LI Ping, BAI Hong-cun, GUO Qing-jie. Molecular model and pyrolysis simulation of Zaoquan coal[J]. J Chem Ind Eng,2019,70(4):1522−1531. [7] 葛涛, WANG Meng, 李芬, 闵凡飞, 张明旭. 高阳炼焦煤碳、氧结构研究与光谱学表征[J]. 煤炭学报,2021,46(3):1024−1031. doi: 10.13225/j.cnki.jccs.2019.1693GE Tao, WANG Meng, LI Fen, MIN Fan-fei, ZHANG Mingxu. Study and characterize of carbon & oxygen structure of Gaoyang coking coal[J]. J China Coal Soc,2021,46(3):1024−1031. doi: 10.13225/j.cnki.jccs.2019.1693 [8] LI Z K, WEI X Y, YAN H L, ZONG Z M. Insight into the structural features of Zhaotong lignite using multiple techniques[J]. Fuel,2015,153(1):176−182. [9] 张帅, 马汝嘉, 刘路, 张浩, 刘钦甫. 扎鲁特地区无烟煤分子结构特征和模型构建[J]. 煤田地质与勘探,2020,48(1):62−69. doi: 10.3969/j.issn.1001-1986.2020.01.009ZHANG Shuai, MA Ru-jia, LIU Lu, ZHANG Hao, LIU Qin-fu. Molecular structure characteristics and model construction of anthracite in Jarud[J]. Coal Geol Explor,2020,48(1):62−69. doi: 10.3969/j.issn.1001-1986.2020.01.009 [10] MATHEWS J P, CHAFFEE A L. The molecular representations of coal - A review[J]. Fuel,2012,96:1−14. doi: 10.1016/j.fuel.2011.11.025 [11] LIN H L, LI K J, ZHANG X W, WANG H X. Structure characterization and model construction of Indonesian brown coal[J]. Energy Fuels,2016,30(5):3809−3814. doi: 10.1021/acs.energyfuels.5b02696 [12] LIN H L, LIAN J, LIU Y P, XUE Y, YAN S, HAN S, WEI W. Comprehensive study of structure model, pyrolysis and liquefaction behaviour of Heidaigou lignite and its liquefied oil[J]. Fuel,2019,240(15):84−91. [13] 相建华, 曾凡桂, 李彬, 张莉, 李美芬, 梁虎珍. 成庄无烟煤大分子结构模型及其分子模拟[J]. 燃料化学学报,2013,41(4):391−399. doi: 10.3969/j.issn.0253-2409.2013.04.002XIANG Jian-hua, ZENG Fan-gui, LI Bin, ZHANG Li, LI Mei-fen, LIANG Hu-zhen. Construction of macromolecular structural model of anthracite from Chengzhuang coal mine and its molecular simulation[J]. J Fuel Chem Technol,2013,41(4):391−399. doi: 10.3969/j.issn.0253-2409.2013.04.002 [14] FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 09[CP]. Wallingford CT: Gaussian Inc., 2009. [15] ZHAO Y, TRUHLAR D G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals[J]. Theor Chem Acc,2008,120:215−241. doi: 10.1007/s00214-007-0310-x [16] 王永刚, 周剑林, 陈艳巨, 胡秀秀, 张书, 林雄超. 13C固体核磁共振分析煤中含氧官能团的研究[J]. 燃料化学学报,2013,41(12):1422−1426.WANG Yong-gang, ZHOU Jian-lin, CHEN Yan-ju, HU Xiu-xiu, ZHANG Shu, LIN Xiong-chao. Contents of O-containing functional groups in coals by 13C NMR analysis[J]. J Fuel Chem Technol,2013,41(12):1422−1426. [17] 陈丽诗, 王岚岚, 潘铁英, 周扬, 张媛媛, 张德详. 固体核磁碳结构参数的修正及其在煤结构分析中的应用[J]. 燃料化学学报,2017,45(10):1153−1163. doi: 10.3969/j.issn.0253-2409.2017.10.001CHEN Li-shi, WANG Lan-lan, PAN Tie-ying, ZHOU Yang, ZHANG Yuan-yuan, ZHANG De-xiang. Calibration of solid state NMR carbon structural parameters and application in coal structure analysis[J]. J Fuel Chem Technol,2017,45(10):1153−1163. doi: 10.3969/j.issn.0253-2409.2017.10.001 [18] DING D, LIU G, FU B. Influence of carbon type on carbon isotopic composition of coal from the perspective of solid-state 13C NMR[J]. Fuel,2019,245(1):174−180. [19] JING Z H, SANDRA R, EKATERINA S, LI M R, WOOD B. Use of FTIR, XPS, NMR to characterize oxidative effects of NaClO on coal molecular structures[J]. Int J Coal Geol,2019,201:1−13. doi: 10.1016/j.coal.2018.11.017 [20] OKOLO G N, NEOMAGUS H W J P, EVERSON R C, ROBERTS M J, BUNT J R, SAKUROVS R, MATHEWS J P. Chemical-structural properties of South African bituminous coals: Insights from wide angle XRD-carbon fraction analysis, ATR-FTIR, solid state 13C NMR, and HRTEM techniques[J]. Fuel,2015,158(15):779−792. [21] WANG Y G, WEI X Y, WANG S K, LI Z K, LI P. Structural evaluation of Xiaolongtan lignite by direct chacracterization and pyrolytic analysis[J]. Fuel Process Technol,2016,144:248−254. doi: 10.1016/j.fuproc.2015.12.034 [22] JIANG J Y, YANG W H, CHENG Y P, LIU Z D, ZHANG Q, ZHAO K. Molecular structure characterization of middle-high rank coal via XRD, Raman and FTIR spectroscopy: Implications for coalification[J]. Fuel,2019,239:559−572. doi: 10.1016/j.fuel.2018.11.057 [23] TIAN B, QIAO Y Y, TIAN Y Y, XIE K C, LIU Q, ZHOU H F. FTIR study on structural changes of different-rank coals caused by single/multiple extraction with cyclohexanone and NMP/CS2 mixed solvent[J]. Fuel Process Technol,2016,154:210−218. doi: 10.1016/j.fuproc.2016.08.035 [24] 梁虎珍, 王传格, 曾凡桂, 李美芬, 相建华. 应用红外光谱研究脱灰对伊敏褐煤结构的影响[J]. 燃料化学学报,2014,42(2):129−137.LIANG Hu-zhen, WANG Chuan-ge, ZENG Fan-gui, LI Mei-fen, XIANG Jian-hua. Effect of demineralization on lignite structure from Yinmin coalfield by FT-IR investigation[J]. J Fuel Chem Technol,2014,42(2):129−137. [25] WANG S Q, TANG Y G, SCHOBERT H H, GUO Y N, GAO W C, LIU X K. FTIR and simultaneous TG/MS/FTIR study of Late Permian coals from Southern China[J]. J Anal Appl Pyrolysis,2013,100:75−80. doi: 10.1016/j.jaap.2012.11.021 [26] GENG W, NAKAJIMA T, TAKANASHI H, OHKI A. Analysis of carboxyl group in coal and coal aromaticity by Fourier transform infrared (FT-IR) spectrometry[J]. Fuel,2009,88(1):139−144. doi: 10.1016/j.fuel.2008.07.027 [27] LIU F R, LI W, GUO H Q, LI B Q, BAI Z Q, HU R S. XPS study on the change of carbon-containing groups and sulfur transformation on coal surface[J]. J Fuel Chem Technol,2011,39(2):81−84. doi: 10.1016/S1872-5813(11)60011-X [28] 葛涛, 张明旭, 马祥梅. 新阳炼焦煤结构的FTIR和XPS谱学表征[J]. 光谱学与光谱分析,2017,37(8):2146−2152.GE Tao, ZHANG Ming-xu, MA Xiang-mei. XPS and FTIR spectroscopy characterization about the structure of coking coal in Xinyang[J]. Spectrosc Spect Anal,2017,37(8):2146−2152. [29] LIU F J, WEI X Y, GUI J, LI P, WANG Y G, LI W T, ZONG Z M, FAN X, ZHAO Y P. Characterization of organonitrogen species in Xianfeng lignite by sequential extraction and ruthenium ion-catalyzed oxidation[J]. Fuel Process Technol,2014,126:199−206. doi: 10.1016/j.fuproc.2014.05.004 [30] BUCKLEY A N. Nitrogen functionality in coals and coal-tar pitch determined by X-ray photoelectron spectroscopy[J]. Fuel Process Technol,1994,38(3):165−179. doi: 10.1016/0378-3820(94)90046-9 [31] KELEMEN S R, AFEWORKI M, GORBATY M L, COHEN A D. Characterization of organically bound oxygen forms in lignites, peats, and pyrolyzed peats by X-ray photoelectron spectroscopy (XPS) and solid-state 13C NMR methods[J]. Energy Fuels,2002,16(6):1450−1462. doi: 10.1021/ef020050k [32] KOZLOWSKI M. XPS study of reductively and non-reductively modified coals[J]. Fuel,2004,83:259−265. doi: 10.1016/j.fuel.2003.08.004 [33] GENG W H, KUMABE Y, NAKAJIMA T, OHKI A. Analysis of hydrothermally-treated and weathered coals by X-ray photoelectron spectroscopy (XPS)[J]. Fuel,2009,88(4):644−649. doi: 10.1016/j.fuel.2008.09.025 [34] YANG Y C, TAO X X, HE H, XU N, TANG L F, WANG S W, GUO J F, CHEN L, YANG Z. X-ray photoelectron spectroscopy study on the chemical forms of S, C and O in coal before and after microwave desulphurization[J]. Int J Oil Gas Coal Technol,2017,15(3):267−286. doi: 10.1504/IJOGCT.2017.084444 [35] SHI K Y, GUI X H, TAO X X, LONG J, JI Y H. Macromolecular structural unit construction of Fushun nitric-acid-oxidized coal[J]. Energy Fuels,2015,29(6):3566−3572. doi: 10.1021/ef502859r [36] NOMURA M, ARTOK L, MURATA S, YAMAMOTO A, HAMA H, GAO H, KIDENA K. Structural evaluation of Zao Zhuang coal[J]. Energy Fuels,1998,12(3):512−23. doi: 10.1021/ef9701448 [37] 秦志宏, 卜良辉, 李祥. 基于炼焦煤族组成和结构参数的焦炭质量预测模型及其成焦机理[J]. 燃料化学学报,2018,46(12):1409−1422.QIN Zhi-hong, BU Liang-hui, LI Xiang. Prediction model for coke quality and mechanism based on coking coal composition and structure parameters[J]. J Fuel Chem Technol,2018,46(12):1409−1422. [38] 葛涛, 李洋, WANG Meng, 李芬, 张明旭. 山西高硫气肥煤结构表征与分子模型构建[J]. 光谱学与光谱分析,2020,40(11):3373−3378.GE Tao, LI Yang, WANG Meng, LI Fen, ZHANG Ming-xu. Structural characterization and molecular model construction of gas-fat coal with high sulfur in Shanxi[J]. Spectrosc Spect Anal,2020,40(11):3373−3378. [39] 周星宇, 曾凡桂, 相建华, 邓小鹏, 相兴华. 马脊梁镜煤有机质大分子模型构建及分子模拟[J]. 化工学报,2020,71(4):1802−1811.ZHOU Xing-yu, ZENG Fan-gui, XIANG Jian-hua, DENG Xiao-peng, XIANG Xing-hua. Macromolecular model construction and molecular simulation of organic matter in Majiliang vitrain[J]. J Chem Ind Eng,2020,71(4):1802−1811. [40] 李壮楣, 王艳美, 李平, 李和平, 白红存, 郭庆杰. 宁东红石湾煤大分子模型构建及量子化学计算[J]. 化工学报,2018,69(5):2208−2216.LI Zhuang-mei, WANG Yan-mei, LI Ping, LI He-ping, BAI Hong-cun, GUO Qing-jie. Macromolecular model construction and quantum chemical calculation of Ningdong Hongshiwan coal[J]. J Chem Ind Eng,2018,69(5):2208−2216. -




 下载:
下载: