Preparation and investigation of carbon-based electrocatalysts from different parts of biomass for oxygen reduction reaction
-
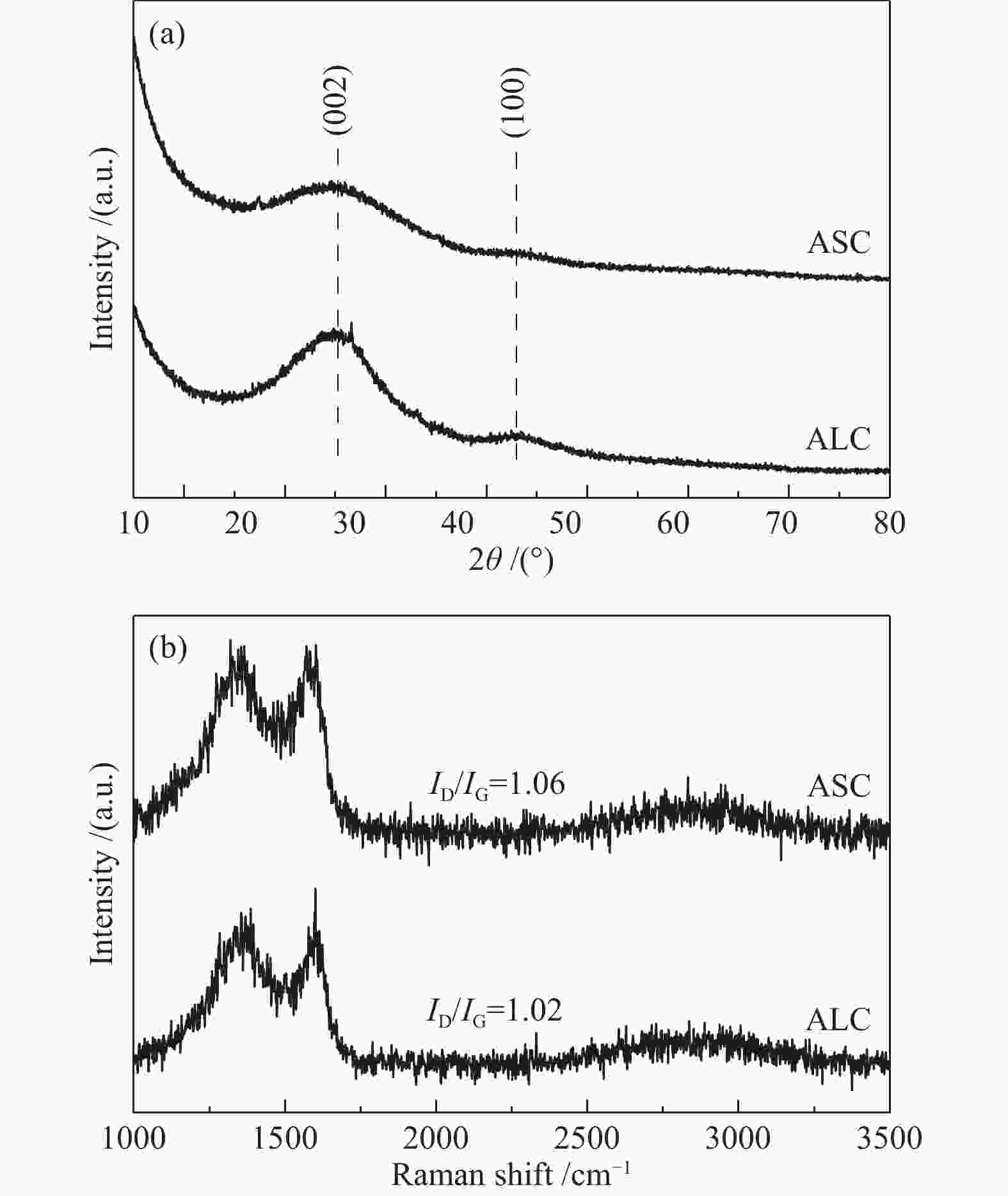
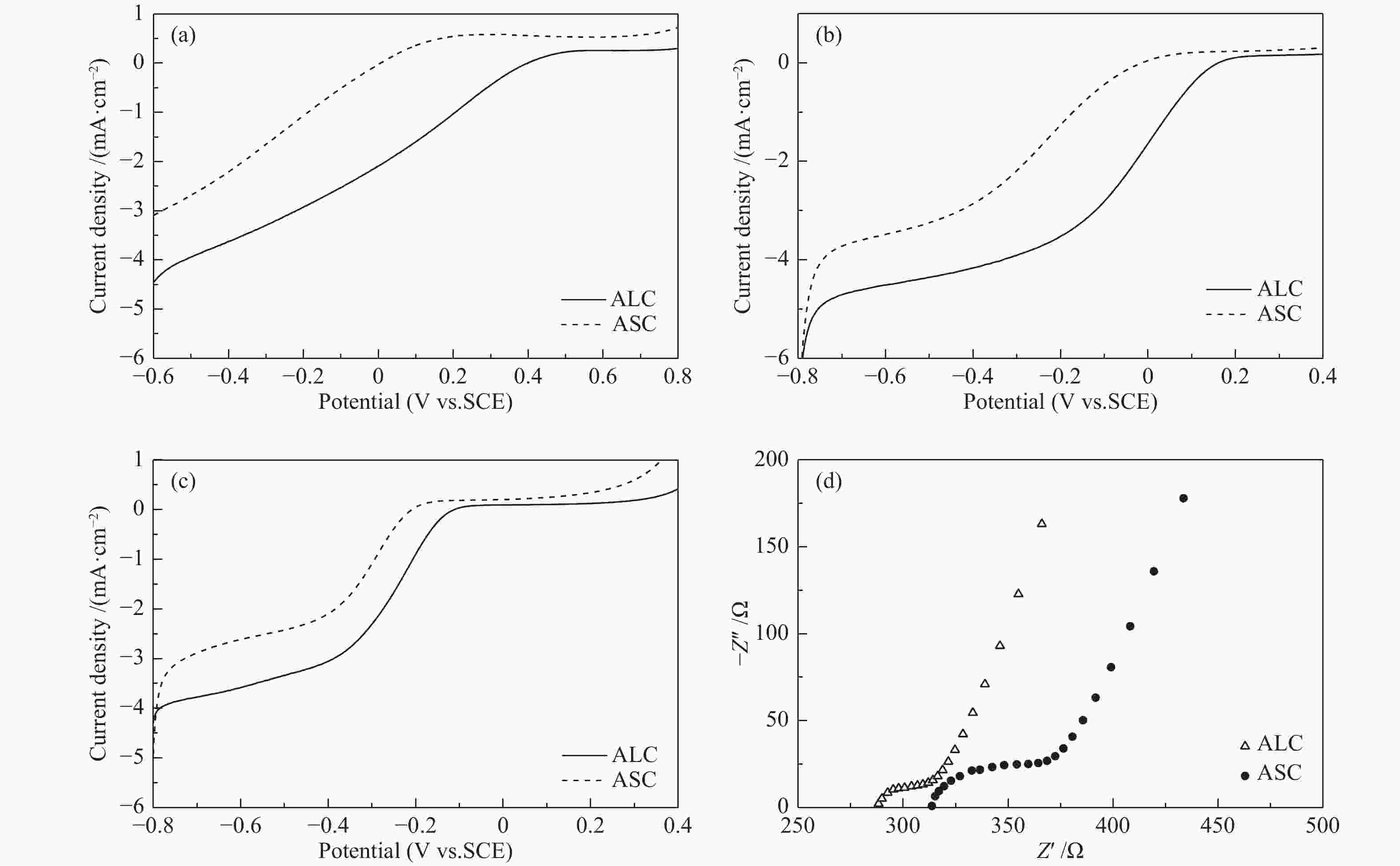
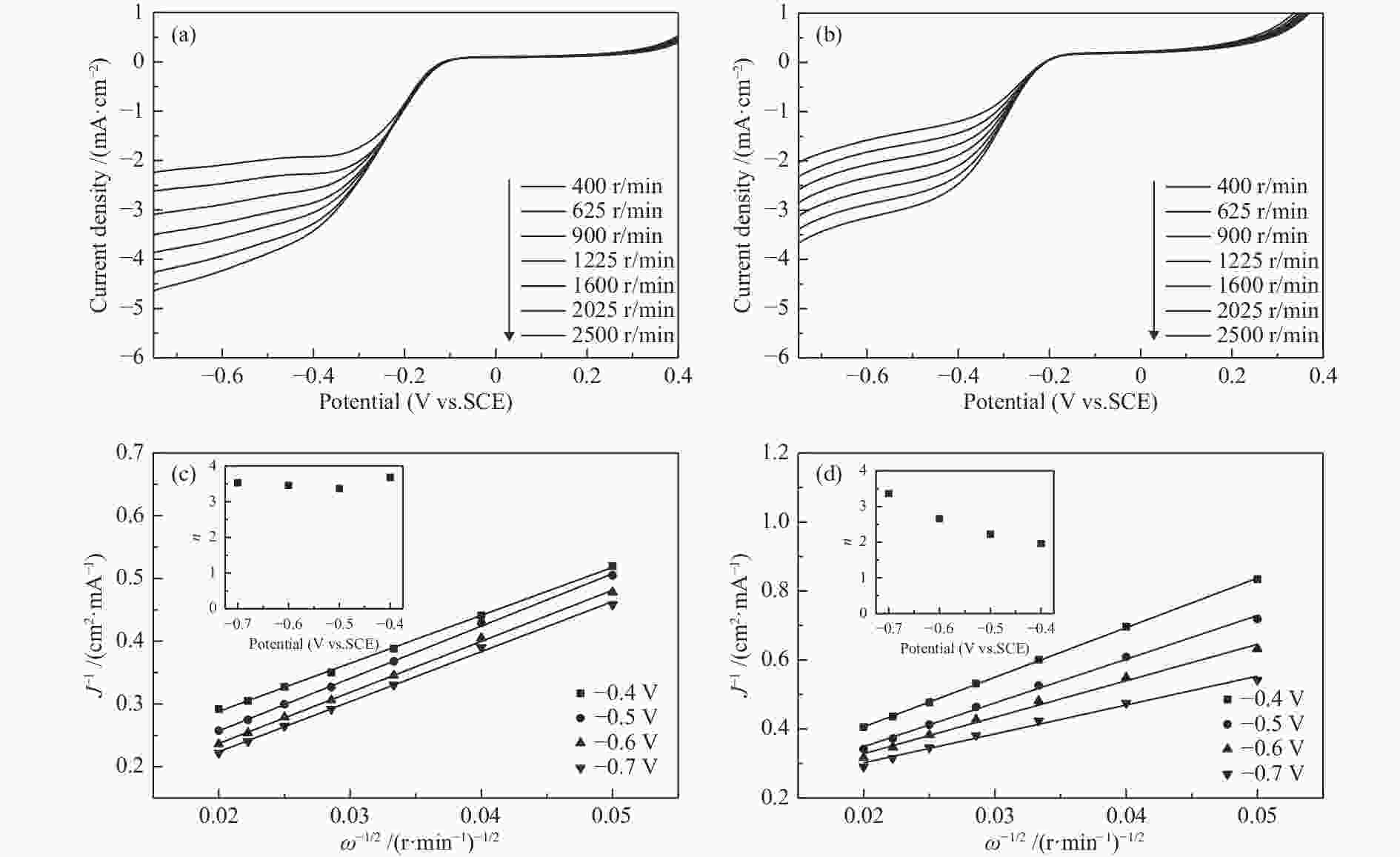
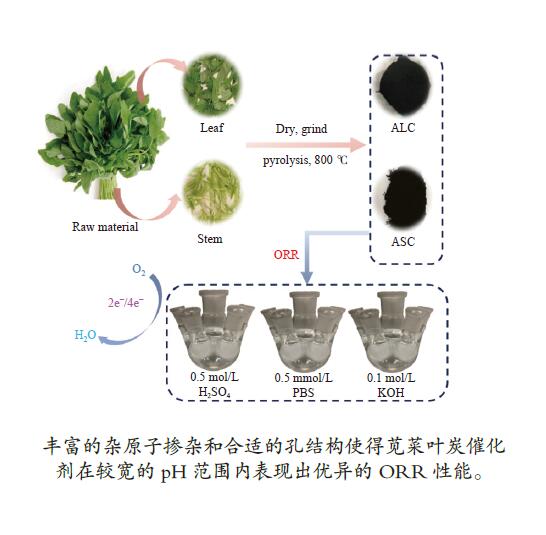
摘要: 炭基氧还原催化剂因其具有成本低、导电性能好,结构可调控、电化学稳定性好等特点在电催化氧还原领域应用广泛。本研究以不同结构(叶、茎)的生物质(苋菜)为原料,通过一步热解法制备炭材料,结合X射线衍射仪、拉曼光谱、X射线光电子能谱以及线性扫描伏安法等物理化学特性分析所制备材料的电催化氧还原反应性能。结果表明,相对于茎源炭,叶源炭(比表面积为732.31 m2/g)的杂原子掺杂更为丰富,特别是其较高的P、S和N共掺杂,尤其是石墨氮和吡啶氮的总含量明显提升。这也使得其在较宽的pH区间(酸性、中性、碱性溶液)表现出优异的氧还原活性(起始还原电位:0.529、0.215、−0.046 V(vs. SCE))。这表明生物质原料自身特性对热解后炭材料的组成、形貌和结构有较大影响,叶源生物炭因其具有更为丰富的杂原子掺杂使得其在催化燃料电池氧还原领域具有广阔的应用前景。Abstract: Carbon-based oxygen reduction catalysts are widely used in catalyzing the oxygen reduction reaction (ORR) due to their low cost, good electrical conductivity, controllable pore structure and good electrochemical stability. In this paper, carbon materials were prepared from different parts of biomass (leaves and stems) through one-step pyrolysis method. The performance of the obtained catalysts was analyzed by X-ray diffraction (XRD), Raman, X-ray Photoelectron Spectroscopy (XPS) and Linear Sweep Voltammetry (LSV). The results demonstrate that compared with the stem carbon, the leaf carbon contains high contents of P, S and N, especially quaternary-N and pyridinic-N, which are responsible for the high performance in the ORR with onset potentials of 0.529, 0.215 and −0.046 V (vs. SCE) in wide ranges of pH (acid, alkali and neutral solutions, respectively). This indicates that biomass based carbon material especially leaf based shows a great application potential for catalyzing ORR in fuel cells.
-
表 1 元素分析和XPS分析
Table 1 Elemental and XPS analysis of the samples
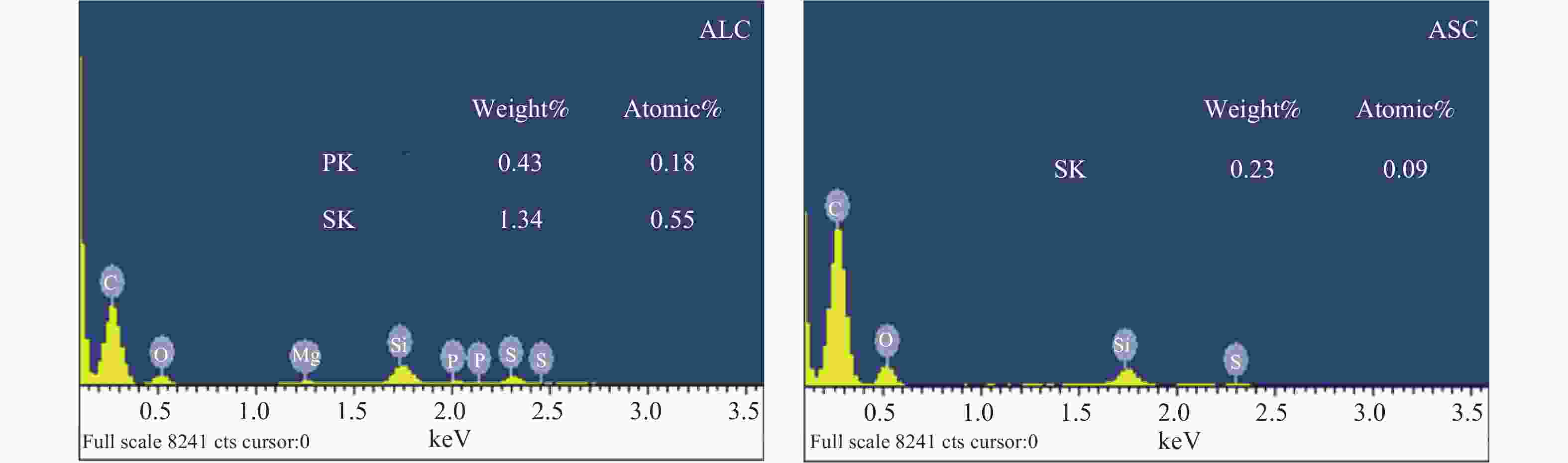
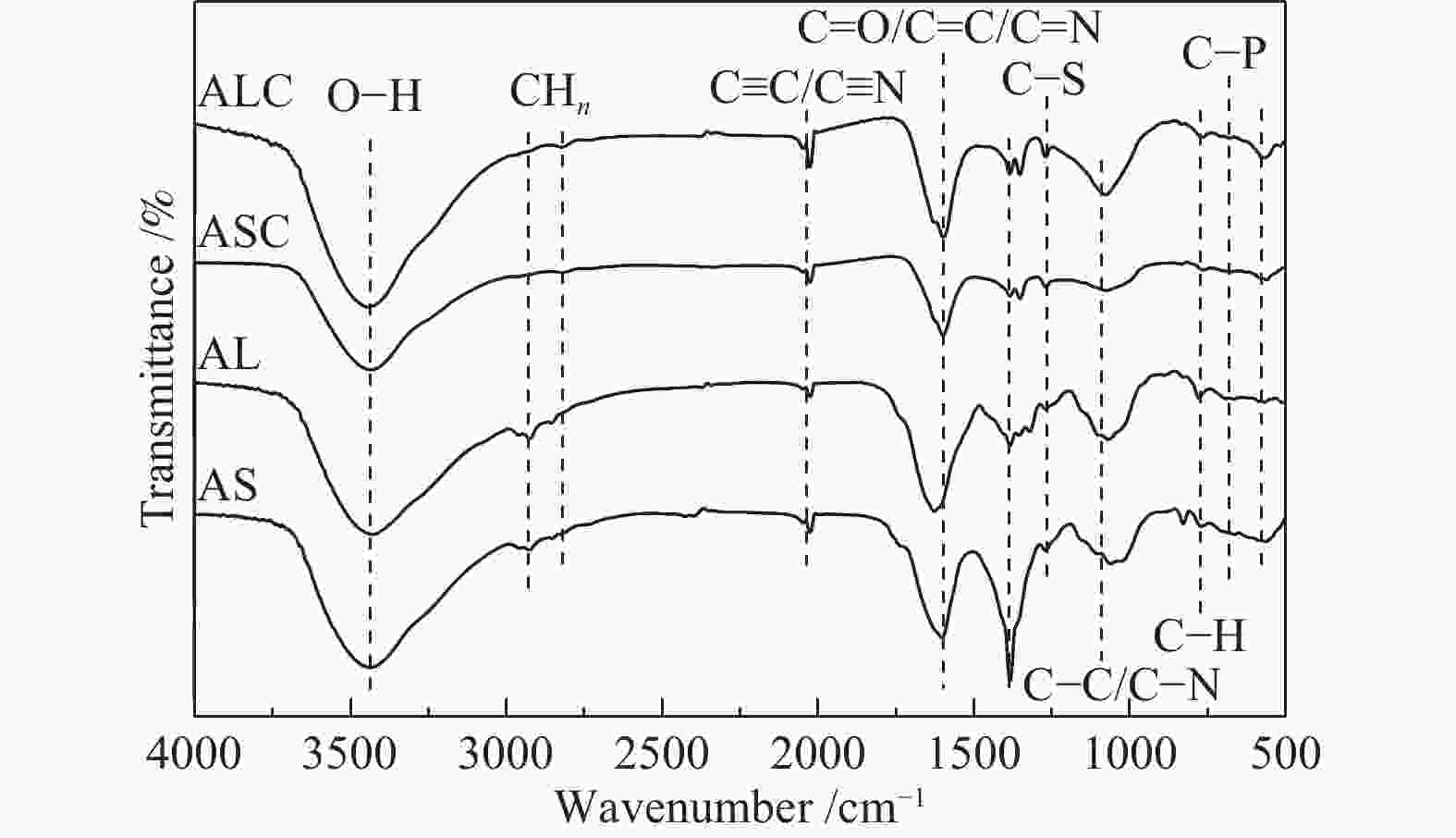
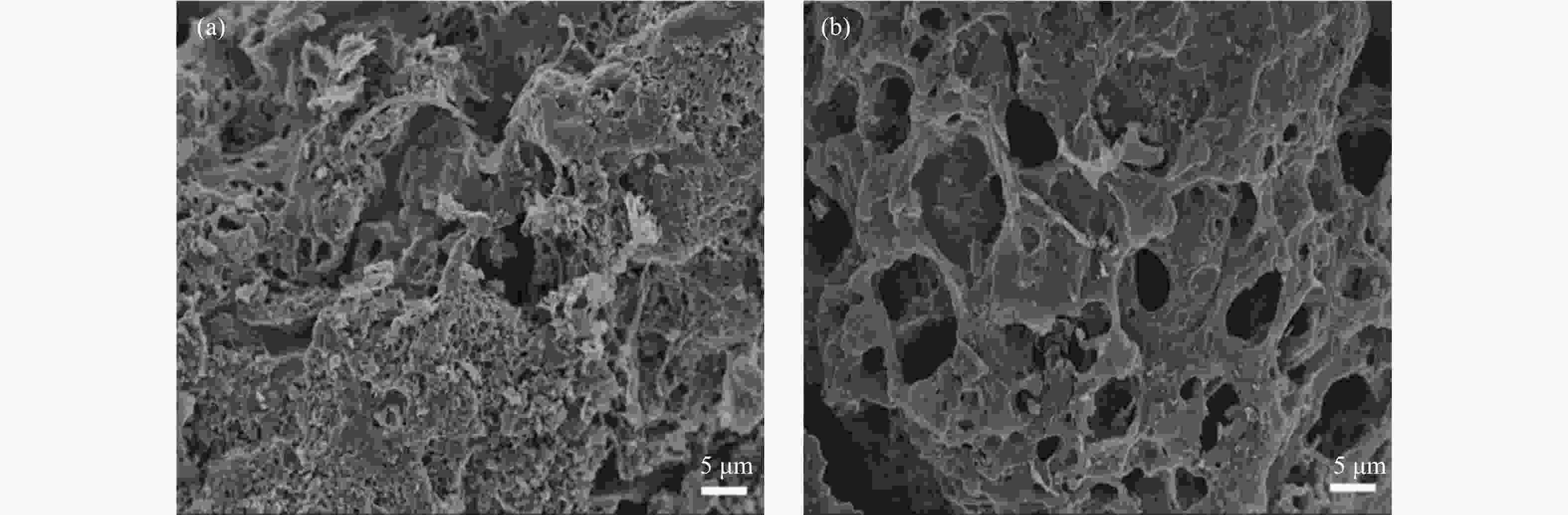
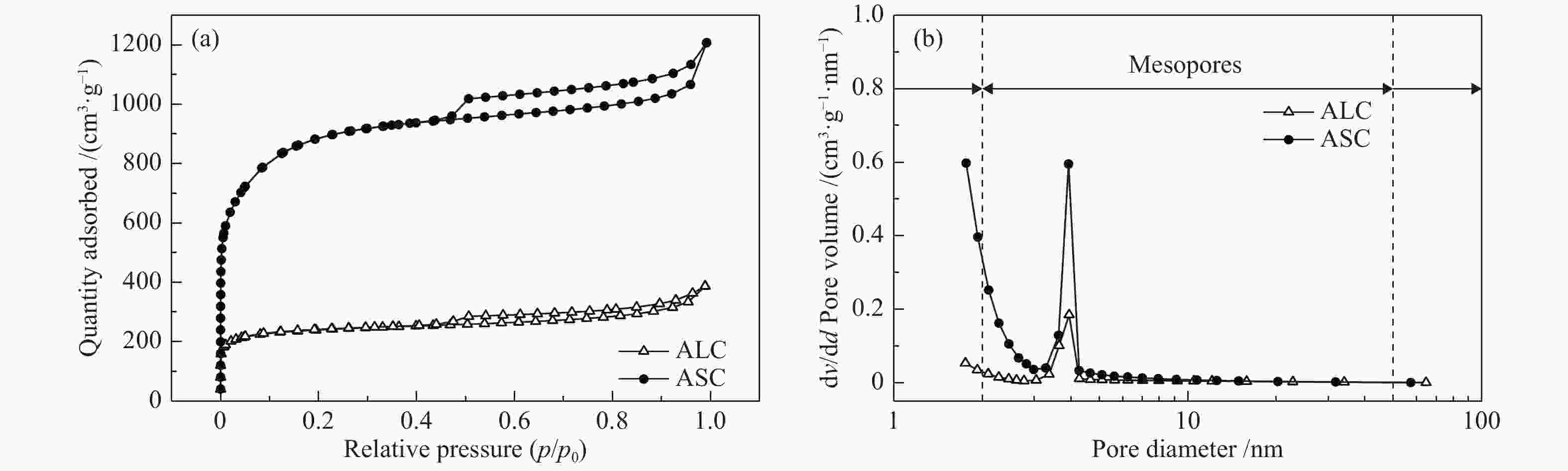
Sample Elemental analysis w/% XPS analysis/% C H N O C N O AL 38.17 5.44 5.94 50.45 53.34 5.80 40.86 AS 29.77 4.45 5.79 59.99 48.89 5.43 45.68 ALC 61.24 1.53 3.63 33.60 72.68 5.20 22.11 ASC 80.54 2.03 2.22 15.21 70.31 3.89 27.22 表 2 ALC和ASC的比表面积及孔容参数
Table 2 Pore parameters of the ALC and ASC
Sample SBET/
(m2·g−1)Smicro/
(m2·g−1)vtotal/
(cm3·g−1)vmirco/
(cm3·g−1)ALC 732.3 504.7 0.51 0.27 ASC 2749.3 1221.2 1.61 0.69 -
[1] YANG G, WANG Y, XU L, LI Y, LI L, SUN Y, YUAN Z, TANG Y. Pd nanochains: Controlled synthesis by lysine and application in microbial fuel cells[J]. Chem Eng J,2020,379(1):122230. [2] WANG X H, GONG X B, PENG L, YANG Z, LIU Y. Tubular nitrogen-doped carbon materials derived from green foxtail as a metal-free electrocatalyst in microbial fuel cells for efficient electron generation[J]. Bioelectrochemistry,2019,127:104−112. doi: 10.1016/j.bioelechem.2019.01.009 [3] LIU X J, ZHOU Y C, ZHOU W J, LI L G, HUANG S B, CHEN S W. Biomass-derived nitrogen self-doped porous carbon as effective metal-free catalysts for oxygen reduction reaction[J]. Nanoscale,2015,7(14):6136−6142. doi: 10.1039/C5NR00013K [4] LIU Z W, PENG F, WANG H J, YU H, ZHENG W X, YANG J A. Phosphorus-doped graphite layers with high electrocatalytic activity for the O-2 reduction in an alkaline medium[J]. Angew Chem Int Ed,2011,50(14):3257−3261. doi: 10.1002/anie.201006768 [5] LU Z S, LI S, LIU C, HE C Z, YANG X W, MA D W, XU G L, YANG Z X. Sulfur doped graphene as a promising metal-free electrocatalyst for oxygen reduction reaction: A DFT-D study[J]. Rsc Adv,2017,7(33):20398−20405. doi: 10.1039/C7RA00632B [6] ZHENG F Y, LI R, GE S, XU W R, ZHANG Y. Nitrogen and phosphorus co-doped carbon networks derived from shrimp shells as an efficient oxygen reduction catalyst for microbial fuel cells[J]. J Power Sources,2020,446:227356. doi: 10.1016/j.jpowsour.2019.227356 [7] SONG M Y, PARK H Y, YANG D S, BHATTACHARJYA D, YU J S. Seaweed-derived heteroatom-doped highly porous carbon as an electrocatalyst for the oxygen reduction reaction[J]. ChemSusChem,2014,7(6):1755−1763. doi: 10.1002/cssc.201400049 [8] XIE L J, SUN G H, SU F Y, GUO X Q, KONG Q Q, LI X M, HUANG X H, WAN L, SONG W, LI K X, LV C X, CHEN C M. Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications[J]. J Mater Chem A,2016,4(5):1637−1646. doi: 10.1039/C5TA09043A [9] ZHAO J, LI Y J, WANG G L, WEI T, LIU Z, CHENG K, YE K, ZHU K, CAO D X, FAN Z J. Enabling high-volumetric-energy-density supercapacitors: Designing open, low-tortuosity heteroatom-doped porous carbon-tube bundle electrodes[J]. J Mater Chem A,2017,5(44):23085−93. doi: 10.1039/C7TA07010A [10] CHEN M D, KANG X Y, WUMAIER T, DOU J Q, GAO B, HAN Y, XU G Q, LIU Z Q, ZHANG L. Preparation of activated carbon from cotton stalk and its application in supercapacitor[J]. J Solid State Electrochem,2013,17(4):1005−1012. doi: 10.1007/s10008-012-1946-6 [11] PAN F P, CAO Z Y, ZHAO Q P, LIANG H Y, ZHANG J Y. Nitrogen-doped porous carbon nanosheets made from biomass as highly active electrocatalyst for oxygen reduction reaction[J]. J Power Sources,2014,272:8−15. doi: 10.1016/j.jpowsour.2014.07.180 [12] WANG H, XU Z W, KOHANDEHGHAN A, LI Z, CUI K, TAN X H, STEPHENSON T J, KING'ONDU C K, HOLT C M B, OLSEN B C, TAK J K, HARFIELD D, ANYIA A O, MITLIN D. Interconnected carbon nanosheets derived from hemp for ultrafast supercapacitors with high energy[J]. ACS Nano,2013,7(6):5131−5141. doi: 10.1021/nn400731g [13] CHEN P, WANG L K, WANG G, GAO M R, GE J, YUAN W J, SHEN Y H, XIE A J, YU S H. Nitrogen-doped nanoporous carbon nanosheets derived from plant biomass: an efficient catalyst for oxygen reduction reaction[J]. Energy Environ Sci,2014,7(12):4095−4103. doi: 10.1039/C4EE02531H [14] LI J, WANG S, REN Y, REN Z, QIU Y, YU J. Nitrogen-doped activated carbon with micrometer-scale channels derived from luffa sponge fibers as electrocatalysts for oxygen reduction reaction with high stability in acidic media[J]. Electrochim Acta,2014,149:56−64. doi: 10.1016/j.electacta.2014.10.089 [15] ESSANDOH M, KUNWAR B, PITTMAN C U, MOHAN D, MLSNA T. Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar[J]. Chem Eng J,2015,265:219−27. doi: 10.1016/j.cej.2014.12.006 [16] 柴明艳. 红苋菜提取物抗氧化活性的研究[J]. 食品研究与开发,2016,37(12):19−23. doi: 10.3969/j.issn.1005-6521.2016.12.005CHAI Ming-yan. Antioxidant activity of extracts from amaranthus tricolor L[J]. Food Res Dev,2016,37(12):19−23. doi: 10.3969/j.issn.1005-6521.2016.12.005 [17] 范晓旭, 杨春花, 周美丽, 杨伟. ICP-MS测定红苋菜根、茎、叶中9种元素含量[J]. 现代食品,2019,(17):150−152, 156.FAN Xiao-xu, YANG Chun-hua, ZHOU Mei-li, YANG Wei. Detection of 9 kinds of elements in root, stem and leaf of red spinach by ICP-MS[J]. Mod Food,2019,(17):150−152, 156. [18] JIANG H L, ZHU Y H, FENG Q, SU Y H, YANG X L, LI C Z. Nitrogen and phosphorus dual-doped hierarchical porous carbon foams as efficient metal-free electrocatalysts for oxygen reduction reactions[J]. Chem-Eur J,2014,20(11):3106−3112. doi: 10.1002/chem.201304561 [19] MENG K, LIU Q, HUANG Y Y, WANG Y B. Facile synthesis of nitrogen and fluorine co-doped carbon materials as efficient electrocatalysts for oxygen reduction reactions in air-cathode microbial fuel cells[J]. J Mater Chem A,2015,3(13):6873−6877. doi: 10.1039/C4TA06500J [20] DIAO R, WANG C, ZHU X F, ZHU X F. Influence of carbonization degree of walnut shell char on pore structure and combustion characteristics[J]. J Fuel Chem Technol,2019,47(10):1173−80. [21] QI J W, ZHANG W D, XU L. Solvent-free mechanochemical preparation of hierarchically porous carbon for supercapacitor and oxygen reduction reaction[J]. Chem-Eur J,2018,24(68):18097−18105. doi: 10.1002/chem.201804302 [22] PENG C, YAN X B, WANG R T, LANG J W, OU Y J, XUE Q J. Promising activated carbons derived from waste tea-leaves and their application in high performance supercapacitors electrodes[J]. Electrochim Acta,2013,87:401−408. doi: 10.1016/j.electacta.2012.09.082 [23] QIANG L L, HU Z G, LI Z M, YANG Y Y, WANG X T, ZHOU Y, ZHANG X Y, WANG W B, WANG Q. Buckwheat husk-derived hierarchical porous nitrogen-doped carbon materials for high-performance symmetric supercapacitor[J]. J Porous Mater,2019,26(4):1217−1225. doi: 10.1007/s10934-019-00723-z [24] LAI L F, POTTS J R, ZHAN D, WANG L, POH C K, TANG C H, GONG H, SHEN Z X, JIANYI L Y, RUOFF R S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction[J]. Energy Environ Sci,2012,5(7):7936−7942. doi: 10.1039/c2ee21802j [25] SUN T T, XU L B, LI S Y, CHAI W X, HUANG Y, YAN Y S, CHEN J F. Cobalt-nitrogen-doped ordered macro-/mesoporous carbon for highly efficient oxygen reduction reaction[J]. Appl Catal B: Environ,2016,193:1−8. doi: 10.1016/j.apcatb.2016.04.006 [26] TAO X, ZHANG Q, LI Y, LV X, MA D, WANG H-G. N, P, S tri-doped hollow carbon nanosphere as a high-efficient bifunctional oxygen electrocatalyst for rechargeable Zn-air batteries[J]. Appl Surf Sci,2019,490:47−55. doi: 10.1016/j.apsusc.2019.06.076 [27] SUN D, BAN R, ZHANG P H, WU G-H, ZHANG J R, ZHU J J. Hair fiber as a precursor for synthesizing of sulfur- and nitrogen-co-doped carbon dots with tunable luminescence properties[J]. Carbon,2013,64:424−34. doi: 10.1016/j.carbon.2013.07.095 [28] WANG X W, SUN G Z, ROUTH P, KIM D H, HUANG W, CHEN P. Heteroatom-doped graphene materials: syntheses, properties and applications[J]. Chem Soc Rev,2014,43(20):7067−7098. doi: 10.1039/C4CS00141A [29] ZHANG L P, NIU J B, LI M T, XIA Z H. Catalytic mechanisms of sulfur-doped graphene as efficient oxygen reduction reaction catalysts for fuel cells[J]. J Phys Chem C,2014,118(7):3545−3553. doi: 10.1021/jp410501u [30] HE D L, ZHAO W, LI P, SUN S, TAN Q W, HAN K, LIU L, LIU L, QU X H. Bifunctional biomass-derived N, S dual-doped ladder-like porous carbon for supercapacitor and oxygen reduction reaction[J]. J Alloy Compd,2019,773:11−20. doi: 10.1016/j.jallcom.2018.09.141 [31] YU Y N, WANG M Q, BAO S J. Biomass-derived synthesis of nitrogen and phosphorus Co-doped mesoporous carbon spheres as catalysts for oxygen reduction reaction[J]. J Solid State Electrochem,2017,21(1):103−110. doi: 10.1007/s10008-016-3346-9 [32] WEI X J, LI Y B, GAO S Y. Biomass-derived interconnected carbon nanoring electrochemical capacitors with high performance in both strongly acidic and alkaline electrolytes[J]. J Mater Chem A,2017,5(1):181−188. doi: 10.1039/C6TA07826E [33] HOU H Y, YU C Y, LIU X X, YAO Y, LIAO Q S, DAI Z P, LI D D. Waste-loofah-derived carbon micro/nanoparticles for lithium ion battery anode[J]. Surf Innov,2018,6(3):159−166. doi: 10.1680/jsuin.17.00068 [34] SONG Y P, ZHAN H, ZHUANG X Z, YIN X L, WU C Z. Synergistic characteristics and capabilities of co-hydrothermal carbonization of sewage sludge/lignite mixtures[J]. Energy Fuels,2019,33(9):8735−8745. doi: 10.1021/acs.energyfuels.9b01766 [35] SUN M, DAVENPORT D, LIU H J, QU J H, ELIMELECH M, LI J H. Highly efficient and sustainable non-precious-metal Fe-N-C electrocatalysts for the oxygen reduction reaction[J]. J Mater Chem A,2018,6(6):2527−2539. doi: 10.1039/C7TA09187G [36] WU J, ZHENG X J, JIN C, TIAN J H, YANG R Z. Ternary doping of phosphorus, nitrogen, and sulfur into porous carbon for enhancing electrocatalytic oxygen reduction[J]. Carbon,2015,92:327−338. doi: 10.1016/j.carbon.2015.05.013 [37] DONG H, YU H, YU H, GAO N, WANG X. Enhanced performance of activated carbon-polytetrafluoroethylene air-cathode by avoidance of sintering on catalyst layer in microbial fuel cells[J]. J Power Sources,2013,232:132−138. doi: 10.1016/j.jpowsour.2013.01.036 [38] XU B, YUE S F, SUI Z Y, ZHANG X T, HOU S S, CAO G P, YANG Y S. What is the choice for supercapacitors: graphene or graphene oxide?[J]. Energy Environ Sci,2011,4(8):2826−30. doi: 10.1039/c1ee01198g [39] LIU J, JIAO M, LU L, BARKHOLTZ H M, LI Y, WANG Y, JIANG L, WU Z, LIU D J, ZHUANG L. High performance platinum single atom electrocatalyst for oxygen reduction reaction[J]. Nat Commun,2017,8:15938. doi: 10.1038/ncomms15938 [40] LIANG J, JIAO Y, JARONIEC M, QIAO S Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance[J]. Angew Chem Int Ed,2012,51(46):11496−11500. doi: 10.1002/anie.201206720 [41] CHOI C H, PARK S H, WOO S I. Phosphorus-nitrogen dual doped carbon as an effective catalyst for oxygen reduction reaction in acidic media: effects of the amount of P-doping on the physical and electrochemical properties of carbon[J]. J Mater Chem,2012,22(24):12107−12115. doi: 10.1039/c2jm31079a -




 下载:
下载: