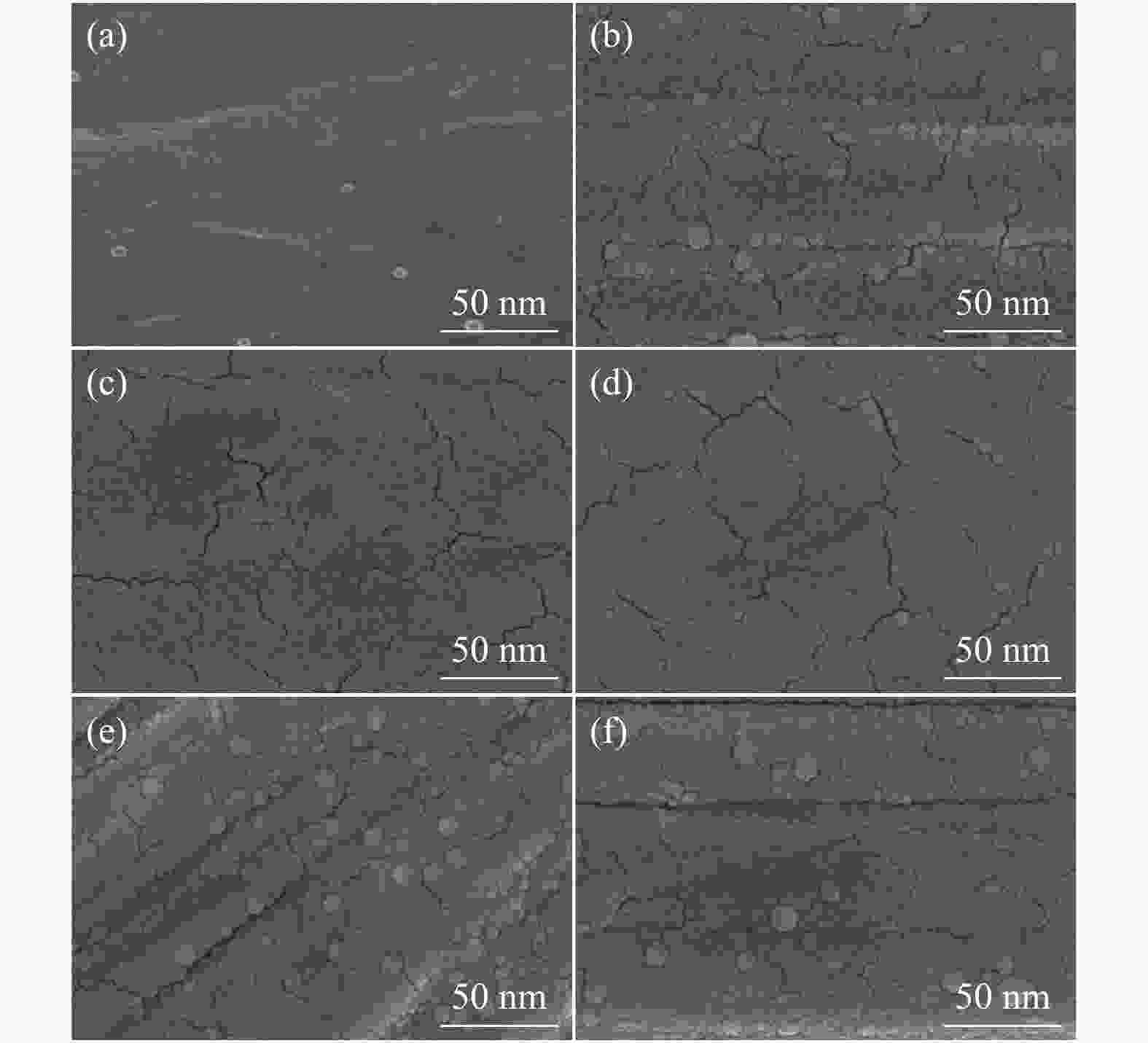
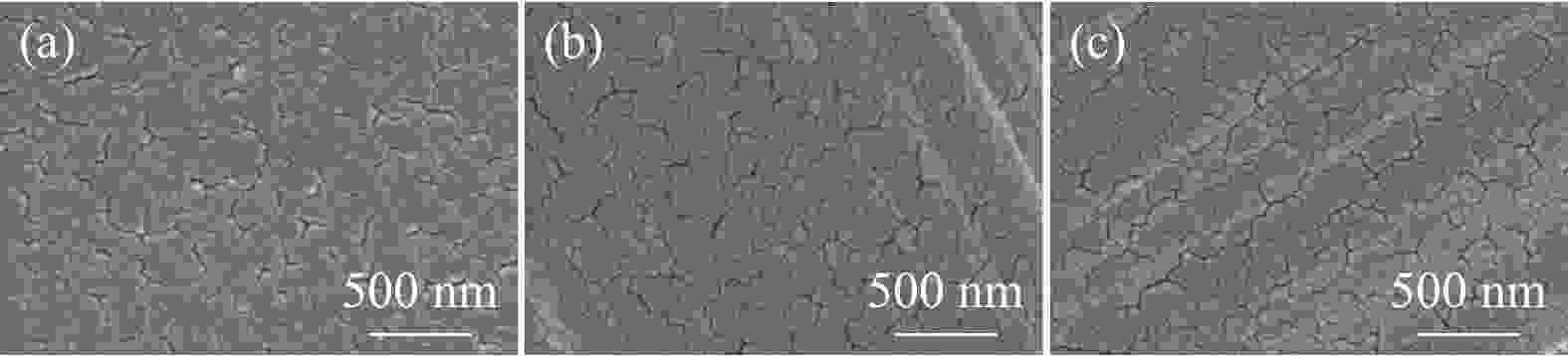
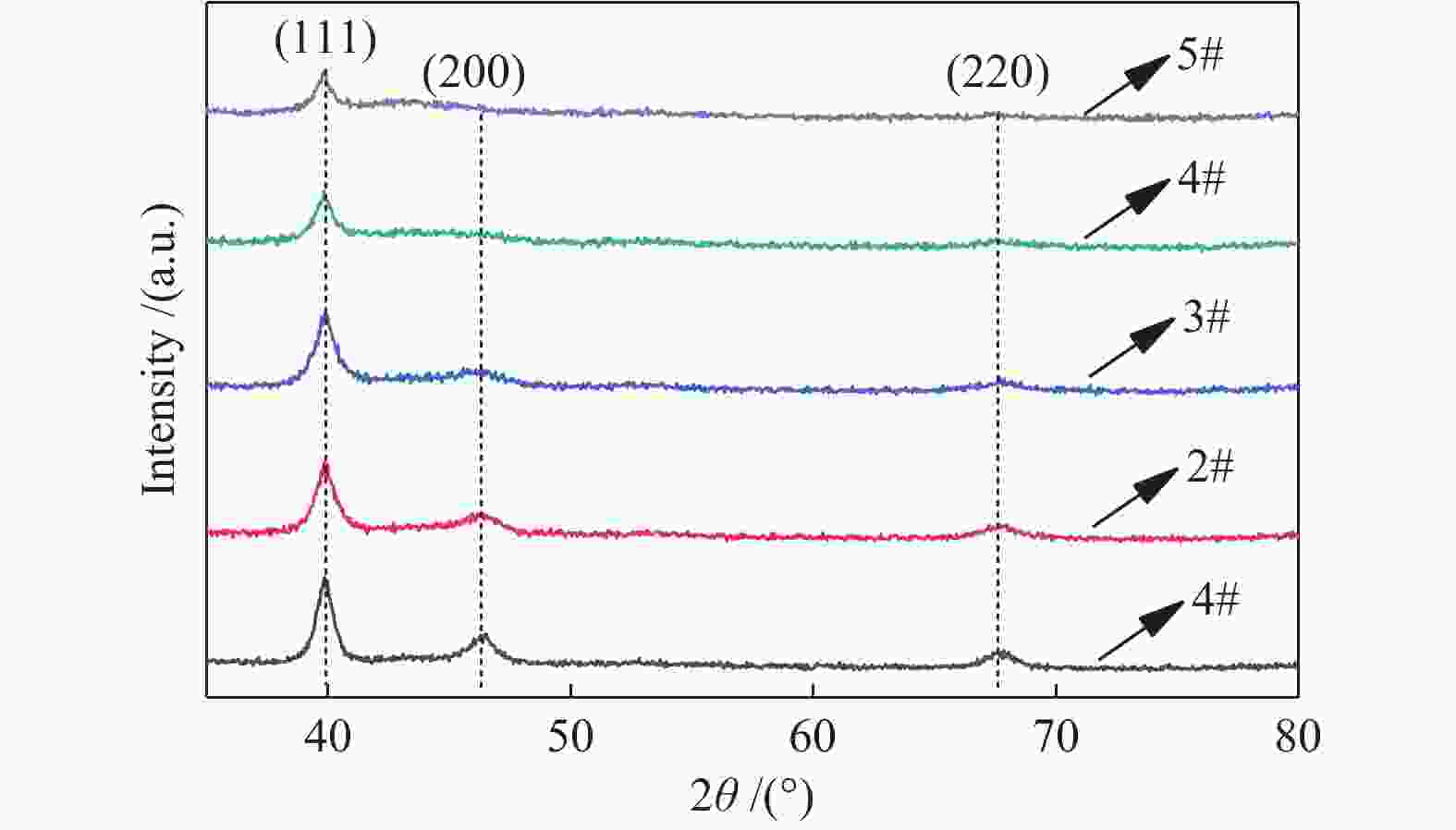
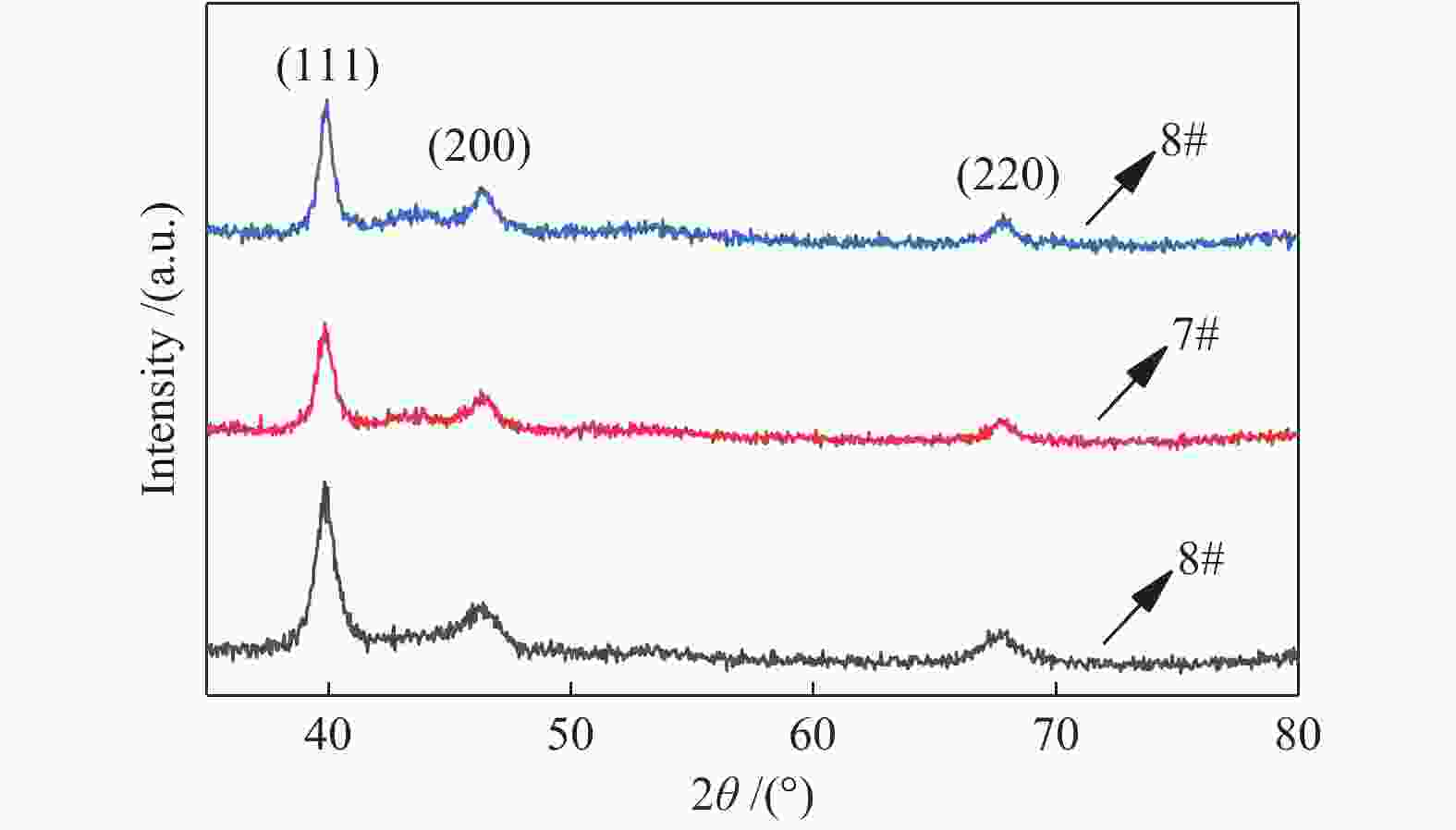
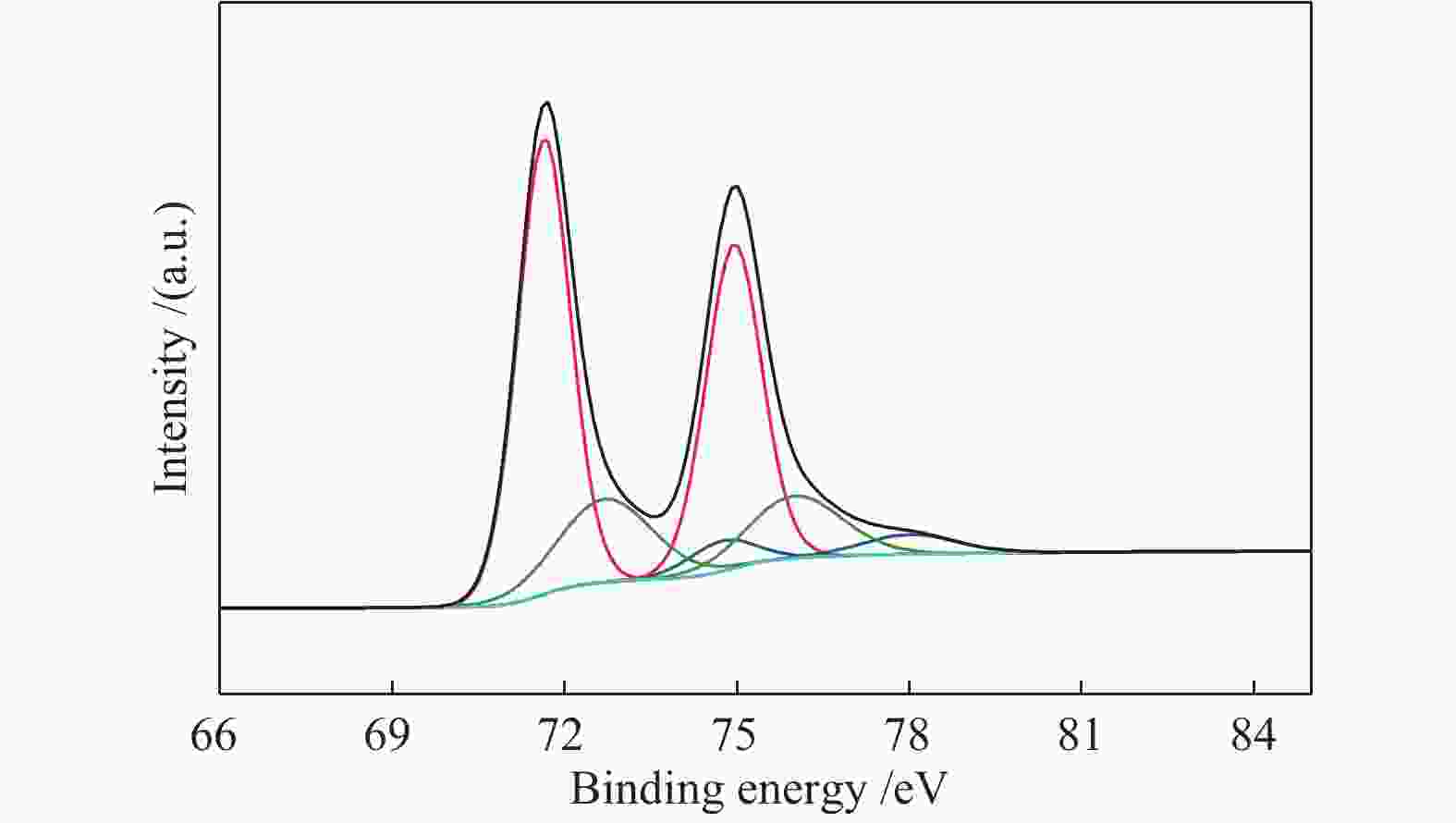
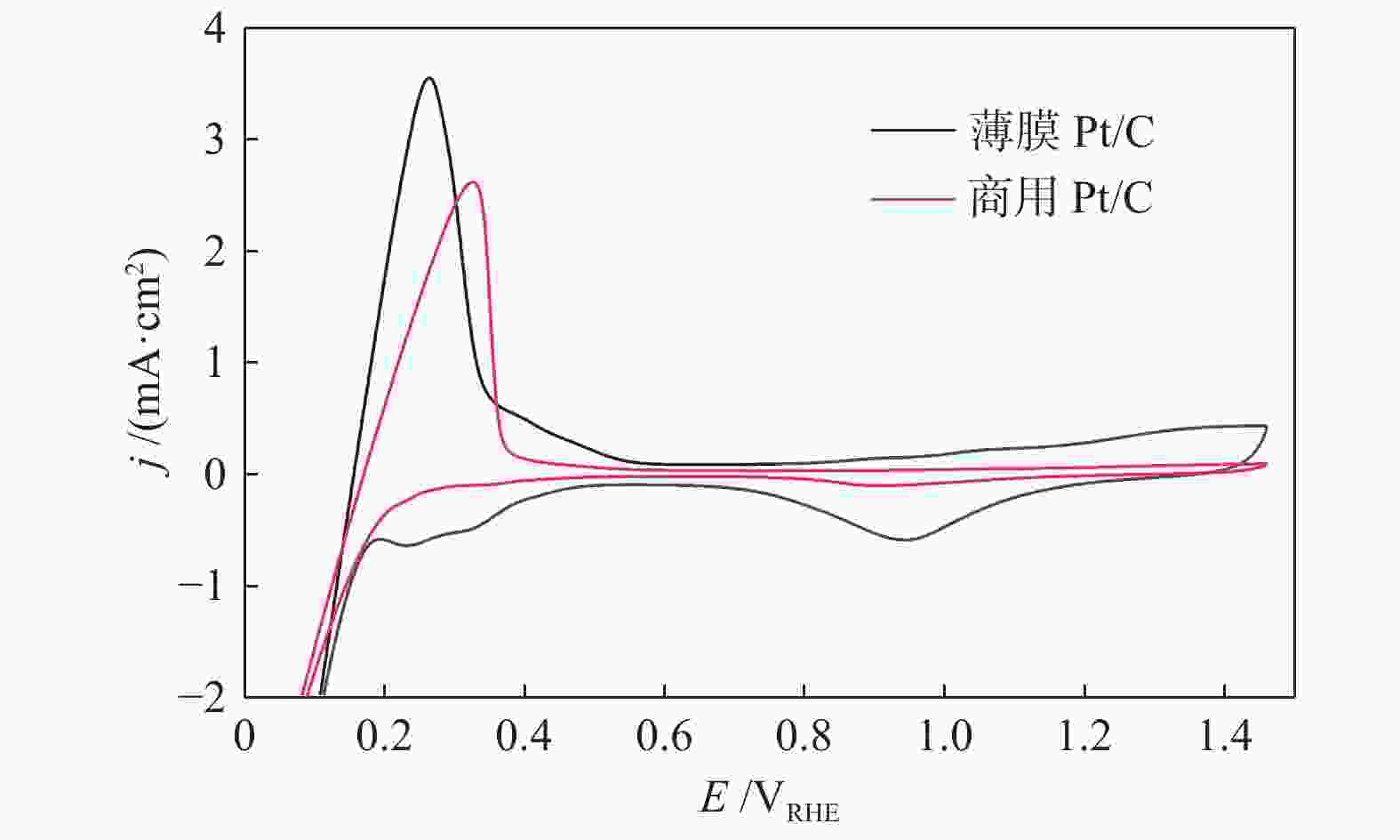
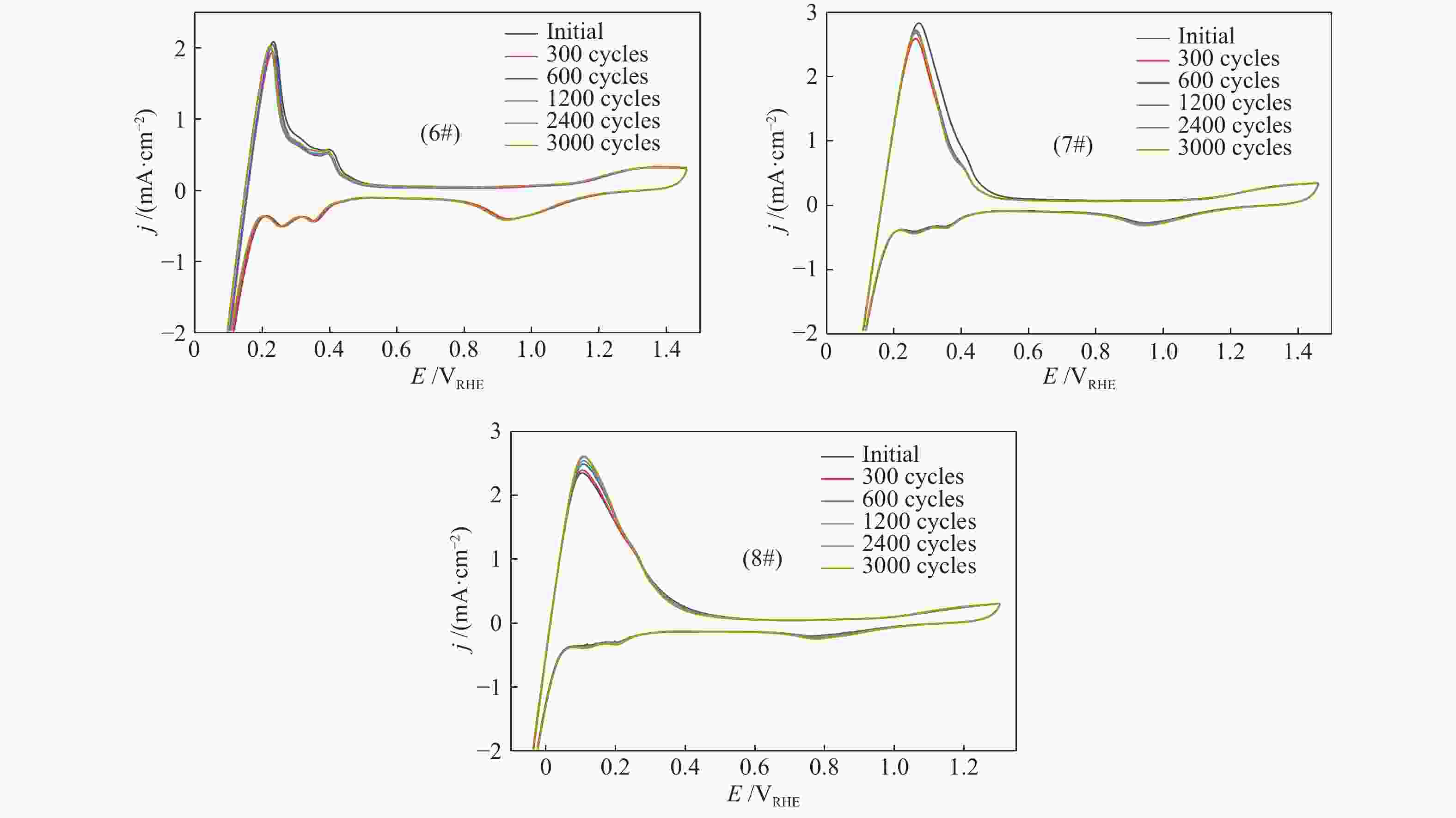
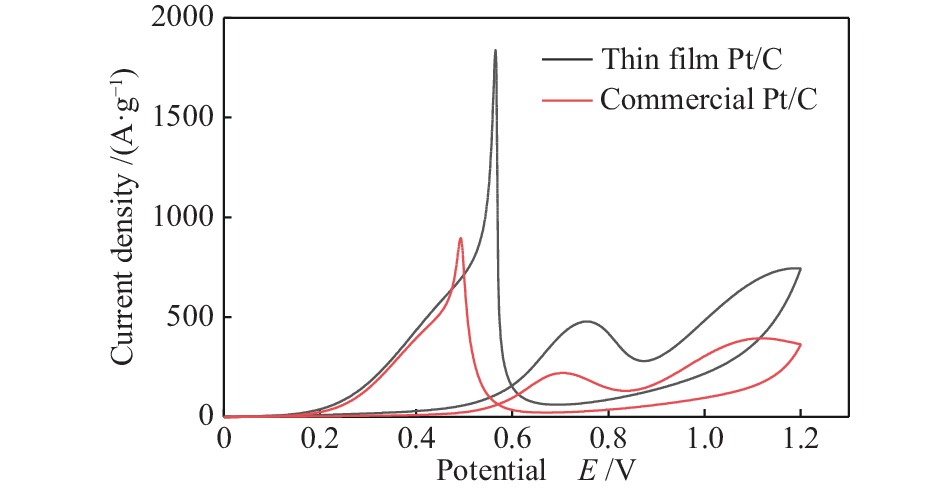
-
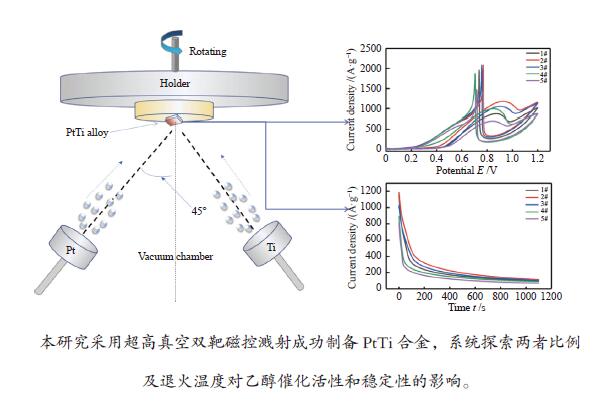
摘要: 以超纯靶材为原料,采用超高真空双靶共溅射系统制备出粒径分布为8.3−12.5 nm,Pt负载量为0.1 mg/cm2的PtTi作为催化剂。利用X射线衍射仪(XRD)、扫描电镜(SEM)、耐用性压力测试(DST)和计时电流(I-t)方法对所制备的PtTi催化剂结构、催化活性及耐久性进行研究,并探究Ti添加量对Pt基合金催化剂电催化性能的影响。结果表明,其最高的电化学活性面积(ECSA)为186.14 m2/g,且经600 ℃原位退火后,直接乙醇催化氧化峰电流密度为1448 A/g,1100 s的稳定电流密度值为147.47 A/g,3000次耐久性压力测试的衰减率为8.6%。本工作研究的催化电极具有优异的催化活性和高稳定性的特性,它可应用于直接乙醇燃料电池(Direct ethanol fuel cell, DEFCs)电极的使用,具有极高的应用潜力。Abstract: PtTi catalysts with particle size distribution of 8.3−12.5 nm and Pt loading capacity of 0.1 mg/cm2 were prepared by ultra-high vacuum dual-target co-sputtering system, and ultra-pure target as raw material. The structure, catalytic activity and durability of the prepared PtTi catalysts were investigated by X-ray diffractometry (XRD), scanning electron microscopy (SEM), durability pressure test (DST) and chronocurrent (I-t) methods, and the effect of the addition amount of Ti on the electrocatalytic performance of Pt-based alloy catalysts was investigated. The results show that the highest electrochemical active area (ECSA) is 186.14 m2/g, and after in-situ annealing at 600 ℃, the peak current density of direct-ethanol catalytic oxidation is 1448 A/g, and the stable current density value of 1100 s is 147.47 A/g, the attenuation rate of 3000 durability stress tests is 8.6%. The catalytic electrode studied in this work has excellent catalytic activity and high stability characteristics. It can be applied to the use of direct ethanol fuel cell electrodes and has extremely high application potential.
-
Key words:
- co-sputtering /
- PtTi catalyst /
- catalytic activity /
- stability /
- ethanol fuel cell
-
图 10 (a)碳纸上共溅射沉积五组催化剂在 1 mol/L CH3CH2OH + 0.5 mol/L H2SO4 溶液中的循环伏安曲线;(b)2#样品经不同温度原位退火后在 1 mol/L CH3CH2OH + 0.5 mol/L H2SO4 溶液中的循环伏安曲线
Figure 10 (a) Cyclic voltammetry curves of 5 groups of catalysts co-sputtered deposited on carbon paper in 1 mol/L CH3CH2OH + 0.5 mol/L H2SO4 solution; (b) Sample 2 is annealed in situ at different temperatures. Cyclic voltammetry curve in mol/L CH3CH2OH + 0.5 mol/L H2SO4 solution
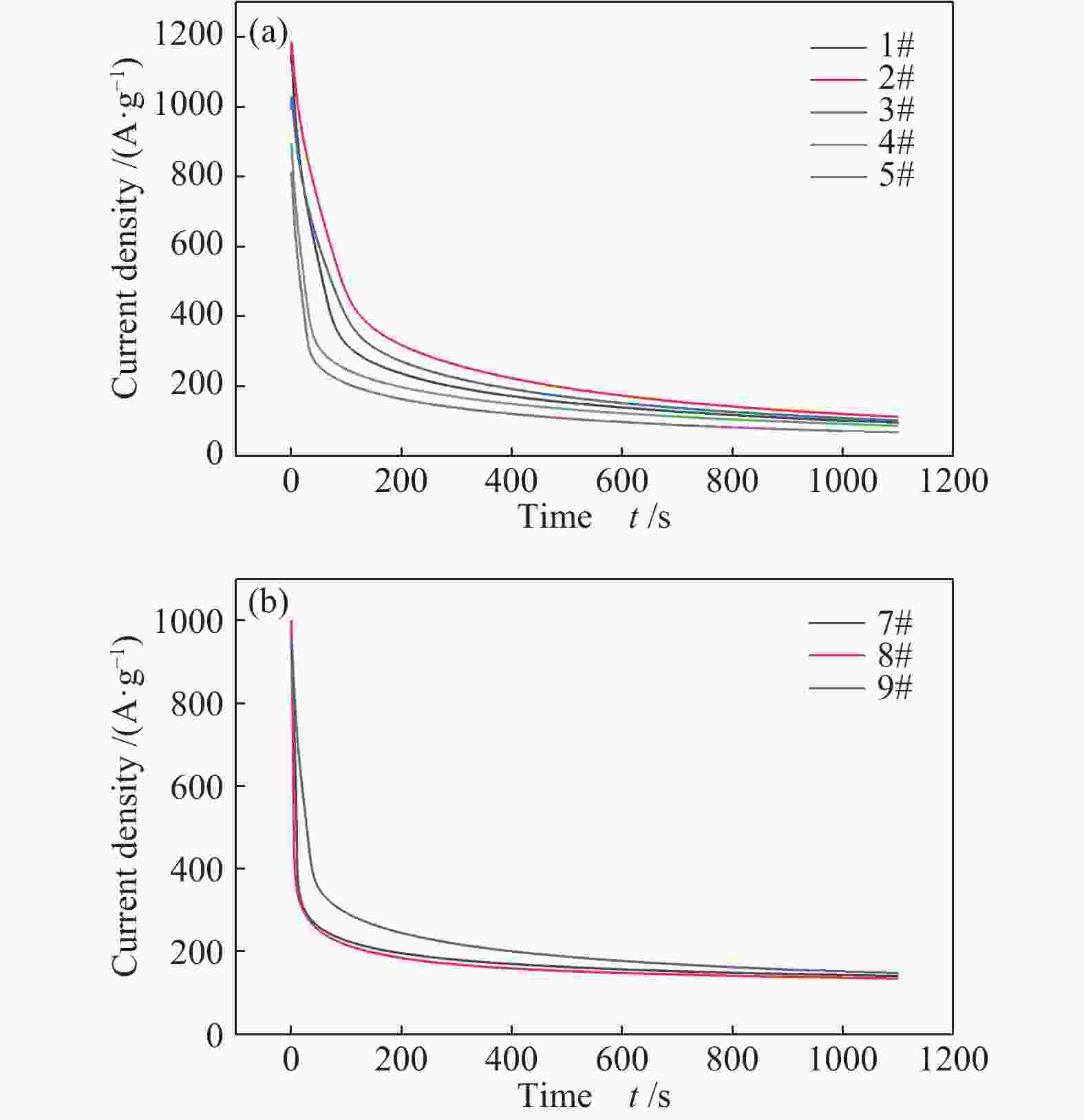
图 12 (a)五组催化剂在 1 mol/L CH3CH2OH + 0.5 mol/L H2SO4 溶液中的 I-t 曲线;(b)不同温度原位退火后三组催化剂在 1 mol/L CH3CH2OH + 0.5 mol/L H2SO4 溶液中的I-t曲线
Figure 12 (a) I-t curves of 5 groups of catalysts in 1 mol/L CH3CH2OH + 0.5 mol/L H2SO4 solution; (b) After in-situ annealing at different temperatures, the 3 groups of catalysts are at 1 mol/L CH3CH2OH + 0.5 mol/L I-t curve in H2SO4 solution
表 1 不同条件的八组催化剂
Table 1 Under different conditions 8 groups of catalysts
Sample 1# 2# 3# 4# 5# 6# 7# 8# PPt/W 30 30 30 30 30 30 30 30 PTi/W 10 20 30 40 50 20 20 20 t/℃ − − − − − 500 600 700 Pt/Ti 9∶1 79∶21 68∶32 57∶43 17∶13 79∶21 79∶21 79∶21 -
[1] LAMY C, BELGSIR E M, LÉGER J M. Electrocatalytic oxidation of aliphatic alcohols: Application to the direct alcohol fuel cell (DAFC)[J]. J Appl Electrochem,2001,31(7):799−809. doi: 10.1023/A:1017587310150 [2] PRAKASH P P, GHADGE S D, JAMPANI H P, KANCHAN D M, BHARAT G, MURUGAVEL S P, KUMTA P N. Active and robust novel bilayer photoanode architectures for hydrogen generation via direct non-electric bias induced photo-electrochemical water splitting[J]. Int J Hydrog Energy,2018,131(43):58−76. [3] RIBEIRO J, ANJOS D M D, KOKOH K B, COUTANCEAU C, LÉGER J M, OLIVI P, ANDRADE A R D, TREMILIOSI-FILHO G. Carbon-supported ternary PtSnIr catalysts for direct ethanol fuel cell[J]. Electrochim Acta,2007,52(24):6997−7006. doi: 10.1016/j.electacta.2007.05.017 [4] YE W, SHOUZHONG Z, WEN-BIN C. Recent advances on electro-oxidation of ethanol on Pt- and Pd-based catalysts: From reaction mechanisms to catalytic materials[J]. Catalysts,2015,5(3):1507−1534. doi: 10.3390/catal5031507 [5] ZHANG J, XING C, SHI F. MoS2/Ti3C2 heterostructure for efficient visible-light photocatalytic hydrogen generation[J]. Int J Hydrogen Energy,2020,45(6):291−301. [6] LAMY C, ROUSSEAU S, BELGSIR E M, COUTANCEAU C, LÉGER J M. Recent progress in the direct ethanol fuel cell: development of new platinum-tin electrocatalysts[J]. Electrochim Acta,2004,49(22/23):3901−3908. doi: 10.1016/j.electacta.2004.01.078 [7] VINCENT I, BESSARABOV D. Low-cost hydrogen production by anion exchange membrane electrolysis: A review[J]. Renewable Sustainable Energy Rev,2018,81(1):690−704. [8] GUO S, DONG S, WANG E. Three-dimensional Pt-on-Pd bimetallic nanodendrites supported on graphene nanosheet: Facile synthesis and used as an advanced nanoelectrocatalyst for methanol oxidation[J]. ACS Nano,2009,4(1):547−555. [9] SCOFIELD M E, KOENIGSMANN C, WANG L, WONG S S. Tailoring the composition of ultrathin, ternary alloy PtRuFe nanowires for the methanol oxidation reaction and formic acid oxidation reaction[J]. Energy Environ Sci,2014,8(1):350−363. [10] RUDI S, GAN L, CUI C, GLIECH M, STRASSER P. Electrochemical dealloying of bimetallic ORR nanoparticle catalysts at constant electrode potentials[J]. J Electrochem Soc,2015,162(4):F403−F409. doi: 10.1149/2.0621504jes [11] ERINI N, KRAUSE P, GLIECH M, YANG R, HUANG Y, STRASSER P. Comparative assessment of synthetic strategies toward active platinum-rhodium-tin electrocatalysts for efficient ethanol electro-oxidation[J]. J Power Sources,2015,294:299−304. [12] RIZO, RUBEN, SEBASTIAN, LÁZARO M J, PASTOR E. On the design of Pt-Sn efficient catalyst for carbon monoxide and ethanol oxidation in acid and alkaline media[J]. Appl Catal B: Environ,2017,200(2):246−254. [13] ANTOLINI E, COLMATI F, GONZALEZ E R. Ethanol oxidation on carbon-supported (PtSn)alloy/SnO2 and (PtSnPd)alloy/SnO2 catalysts with a fixed Pt/SnO2 atomic ratio: Effect of the alloy phase characteristics[J]. J Power Sources,2009,193(2):555−561. doi: 10.1016/j.jpowsour.2009.04.039 [14] SPENDELOW J S, WIECKOWSKI A. Electrocatalysis of oxygen reduction and small alcohol oxidation in alkaline media[J]. Phys Chem Chem Phys,2007,9(21):2654−2675. doi: 10.1039/b703315j [15] VINCENT I, KRUGER A, BESSARABOV D. Development of an efficient mem-brane electrode assembly for low-cost hydrogen production by anion exchange membrane electrolysis[J]. Int J Hydrog Energy,2017,42(107):52−61. [16] VALERIO NETO, EDMUNDO S, GOMES, SALAZAR-BANDA G R, EGUILUZ K I B. Pt and Pt-Rh nanowires supported on carbon and SnO2: Sb nanoparticles for ethanol electrochemical oxidation in acidic media[J]. Int J Hydrog Energy,2018,43(1):178−188. doi: 10.1016/j.ijhydene.2017.11.014 [17] KATTEL S, DUAN Z, WANG G. Density functional theory study of an oxygen reduction reaction on a Pt3Ti alloy electrocatalyst[J]. J Phys Chem C,2013,117(14):7107−7113. doi: 10.1021/jp400158r [18] ZHOU Q, XU C. Stratified nanoporous PtTi alloys for hydrolysis of ammonia borane[J]. J Colloid Interface Sci,2017,496:235−242. doi: 10.1016/j.jcis.2017.02.030 [19] HOGARTH M P, RALPH T R. Catalysis for low-temperature fuel cells[J]. Platin Met Rev,2002,46(4):146−164. [20] OZTURK O, OZDEMIR O K, ULUSOY I, AHSEN A S, SLAUCHEVA E. Effect of Ti sublayer on the ORR catalytic efficiency of dc magnetron sputtered thin Pt films[J]. Int J Hydrog Energy,2010,35(10):4466−4473. doi: 10.1016/j.ijhydene.2010.02.077 [21] SIEVERS G, MUELLER S, QUADE A, STEFFEN F, JAKUBITH S, KRUTH A, BRUESER V. Mesoporous Pt-Co oxygen reduction reaction (ORR) catalysts for low temperature proton exchange membrane fuel cell synthesized by alternating sputtering[J]. J Power Sources,2014,268(dec. 5):255−260. [22] OSTROVERKH A, DUBAU M, JOHÁNEK V, VÁCLAV M, MÍD B, VELTRUSKÁ K, OSTROVERKH Y, FIALA R, MATOLÍN V. Efficient Pt‐C MEA for pemfc with low platinum content prepared by magnetron sputtering[J]. Fuel Cells,2018,18(1):51−56. doi: 10.1002/fuce.201700137 [23] MOUGENOT M, CAILLARD A, BRAULT P, BARANTON S, COUTANCEAU C. High-performance plasma sputtered PdPt fuel cell electrodes with ultra-low loading[J]. Int J Hydrog Energy,2011,36(14):8429−8434. doi: 10.1016/j.ijhydene.2011.04.080 [24] LI Y H, HONG J R. Performance assessment of catalytic combustion-driven thermophotovoltaic platinum tubular reactor[J]. ACS Appl Energy Mater,2018,211(84):3−53. [25] SEGER B, KAMAT P V. Electrocatalytically active graphene-platinum nanocomposites. role of 2-D carbon support in PEM fuel cells[J]. J Phys Chem C,2009,113(19):7990−7995. doi: 10.1021/jp900360k [26] HSU R S, HIGGINS D, CHEN Z. Tin-oxide-coated single-walled carbon nanotube bundles supporting platinum electrocatalysts for direct ethanol fuel cells[J]. Nanotechnology,2010,21(16):165705. doi: 10.1088/0957-4484/21/16/165705 [27] BELLOSTA VON COLBE J, ARES J-R, BARALE J, BARICCO M, BUCKLEY C, CAPURSO G. Application of hydrides in hydrogen storage and compression: achievements, outlook and perspectives[J]. Int J Hydrog Energy,2019,44(7):780−808. [28] PHILLIPS R, DUNNILL C W. Zero gap alkaline electrolysis cell design for renewable energy storage as hydrogen gas[J]. RSC Adv,2016,100(6):43−51. [29] MOHSIN M, RASHEED A K, SAIDUR R. Economic viability and production capacity of wind-generated renewable hydrogen[J]. Int J Hydrog Energy,2018,26(43):21−30. [30] OUMA C N, MODISHA P M, BESSARABOV D. Catalytic dehydrogenation of the liquid organic hydrogen carrier octa hydro indole on Pt (111) surface: ab initio insights from density functional theory calculations[J]. Appl Surf Sci,2019,471(10):34−40. [31] SHEN Y, ZHANG M Z, XIAO K, XI J. Synthesis of Pt, PtRh, and PtRhNi alloys supported by pristine graphene nanosheets for ethanol electrooxidation[J]. ChemCatChem,2014,6(11):3254−3261. doi: 10.1002/cctc.201402629 [32] CELEK M S, PINARBAS A. Investigations on performance and emission characteristics of an industrial low swirl burner while burning natural gas, methane, hydrogen-enriched natural gas, and hydrogen as fuels[J]. Int J Hydrog Energy,2018,43(1):194−207. [33] DU PREEZ S P, JONES D R, BESSARABOV D G, FALCH A, MOTA DAS NEVES QUARESMA C, DUNNILL C W. Development of a Pt/stainless steel mesh catalyst and its application in catalytic hydrogen combustion[J]. Int J Hydrog Energy,2019,44(27):94−106. [34] LV Q, XIAO Y, YIN M, GE J, XING W, LIU C. Reconstructed PtFe alloy nanoparticles with bulk-surface differential structure for methanol oxidation[J]. Electrochim Acta,2014,139:61−68. -




 下载:
下载: