Study of CeO2/LaFeO3 in chemical looping reforming of methane for syngas production
-
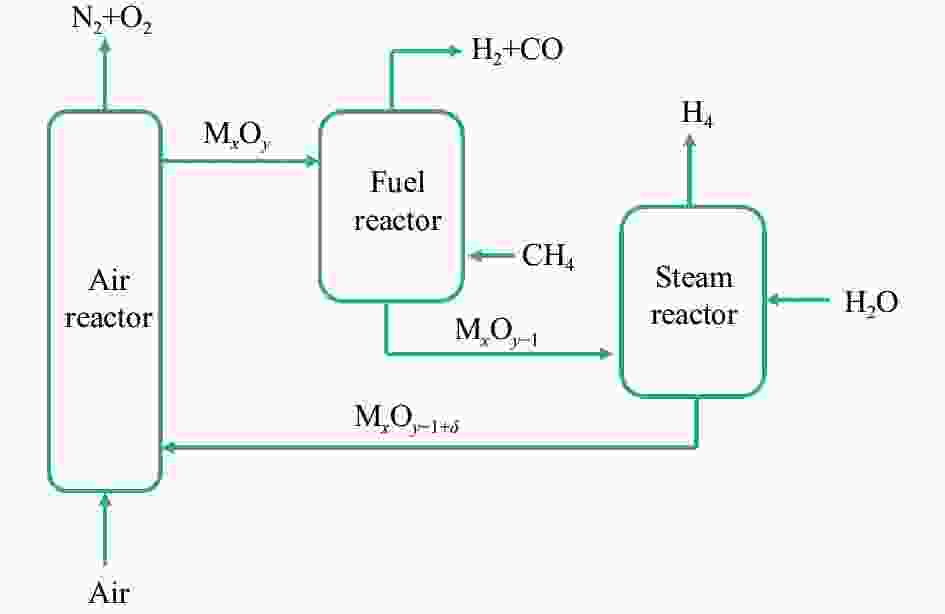
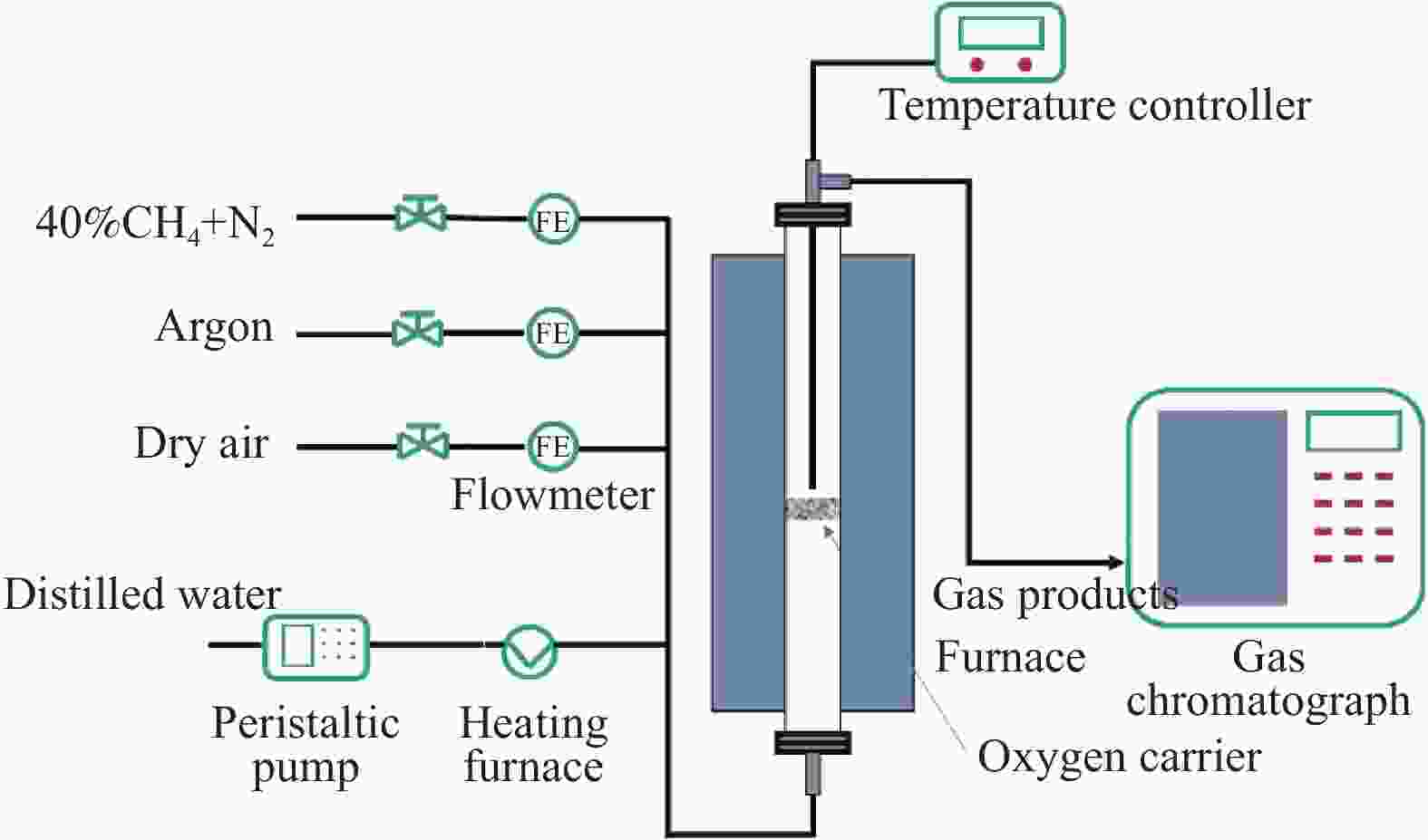
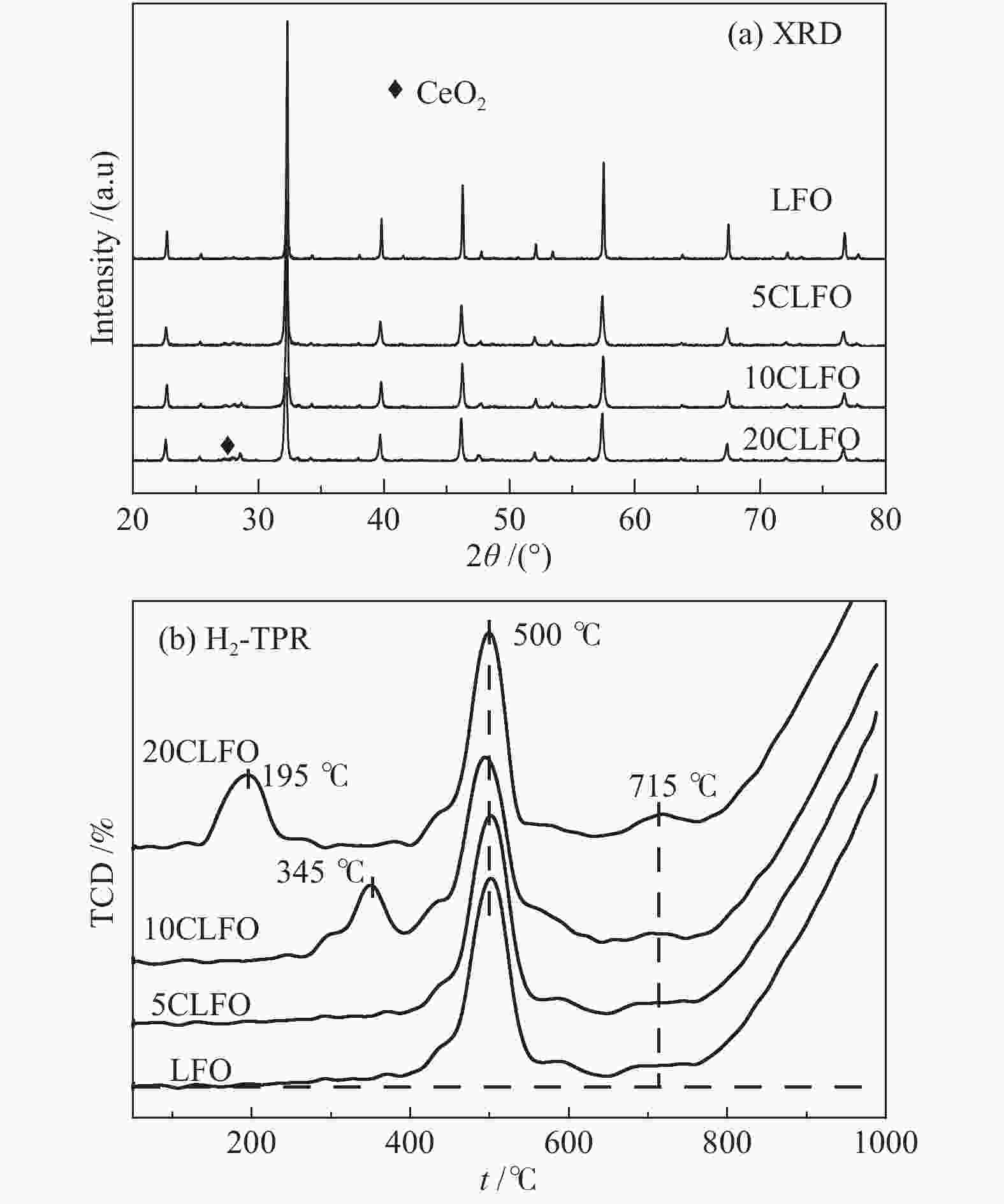
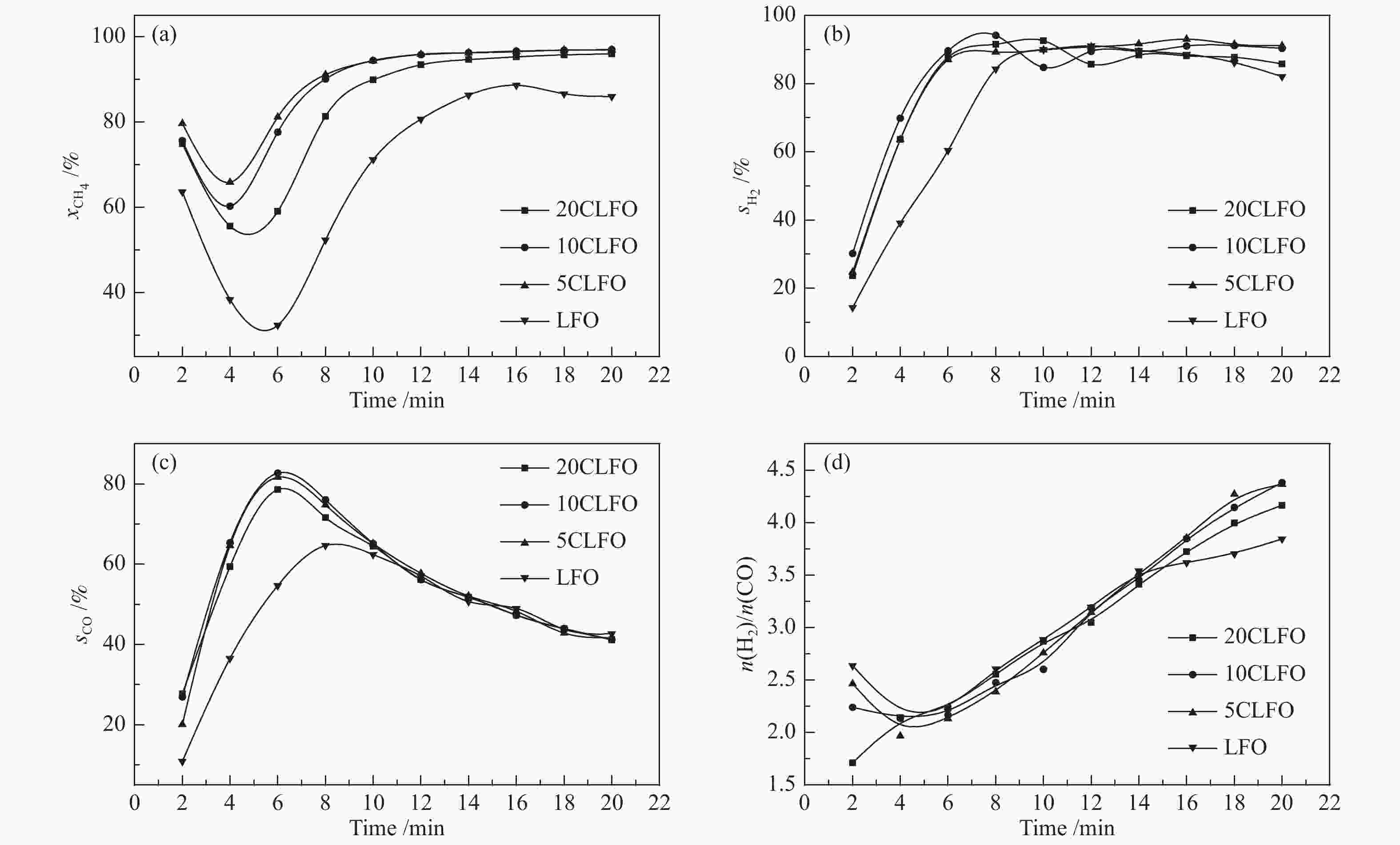
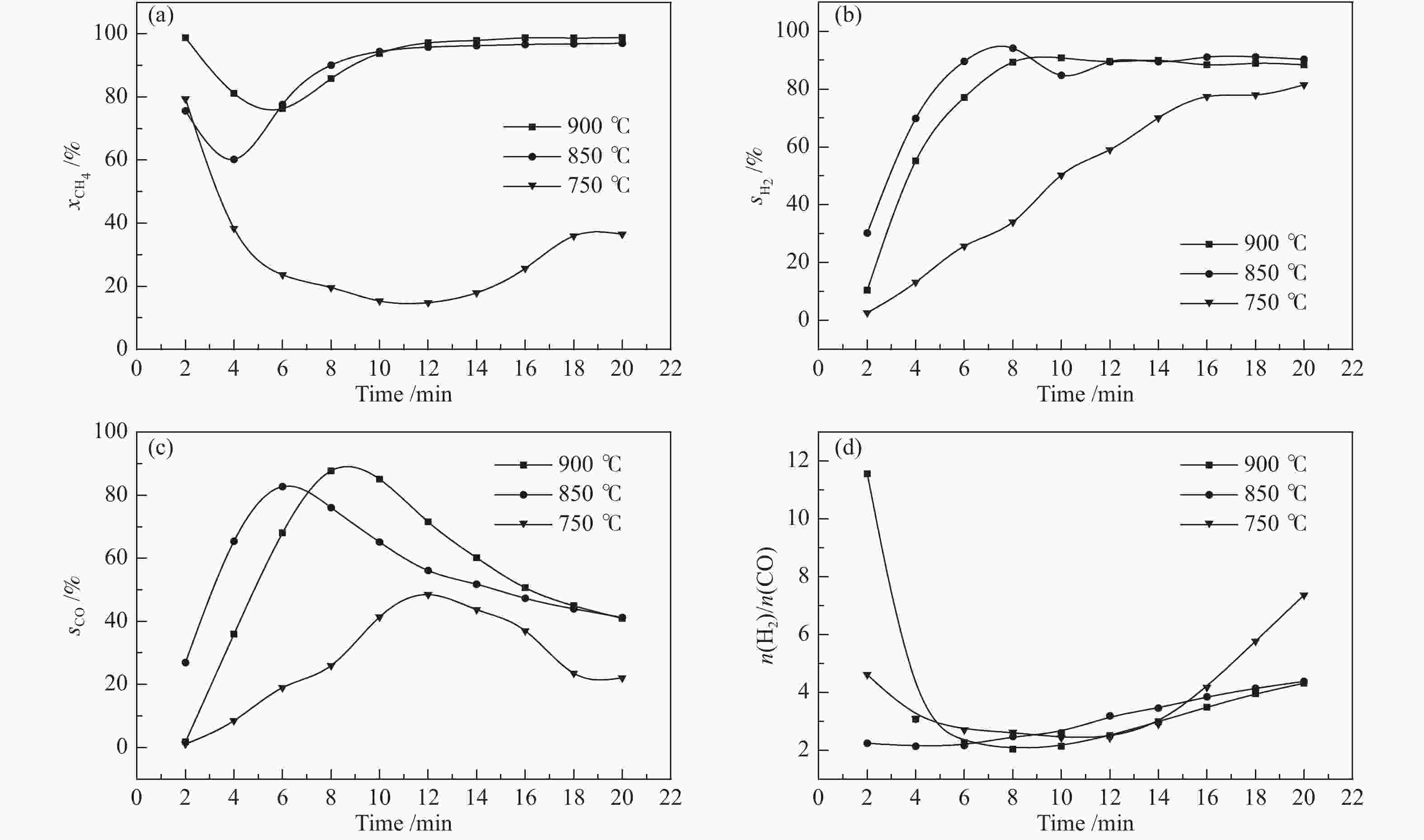
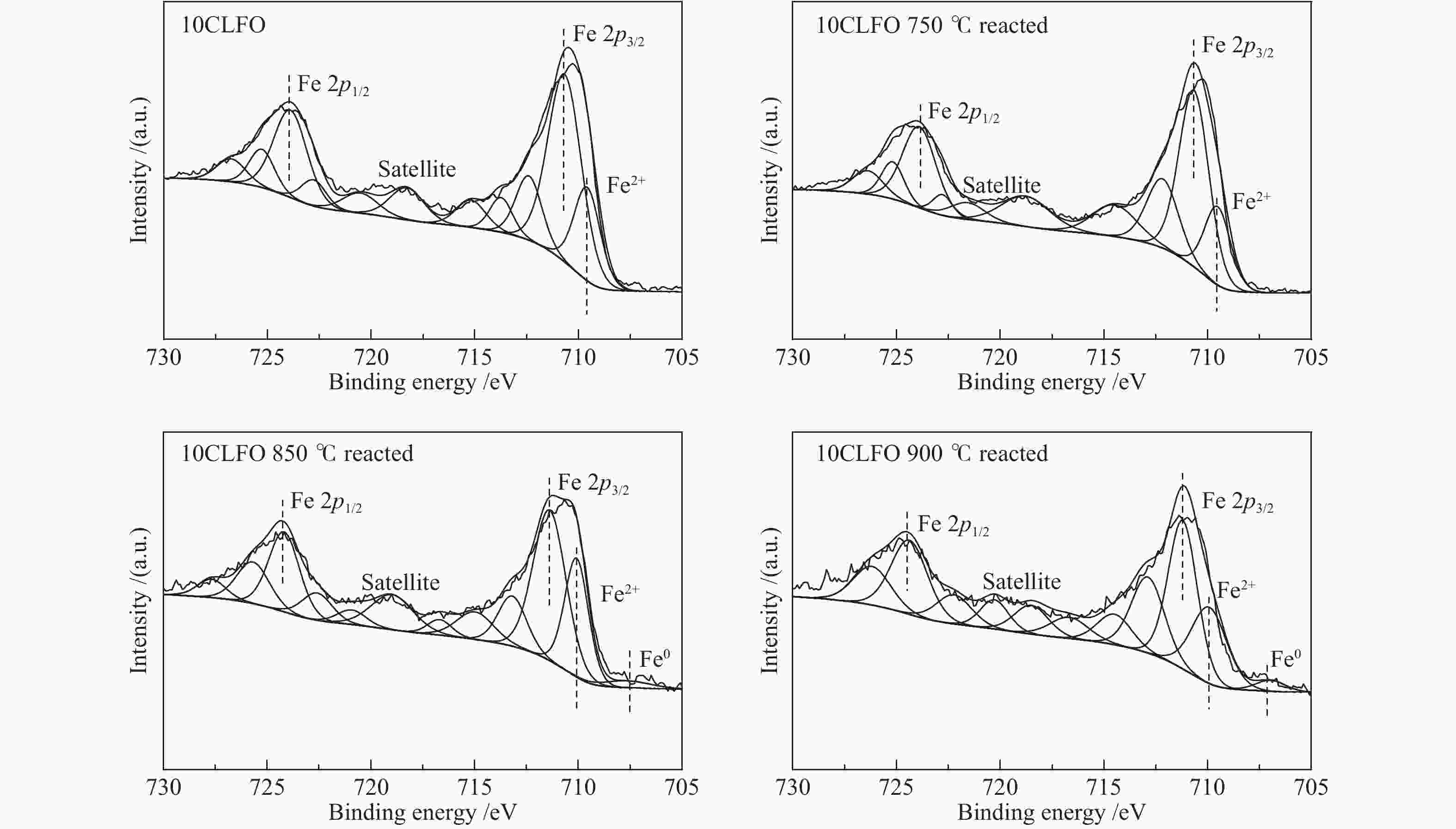
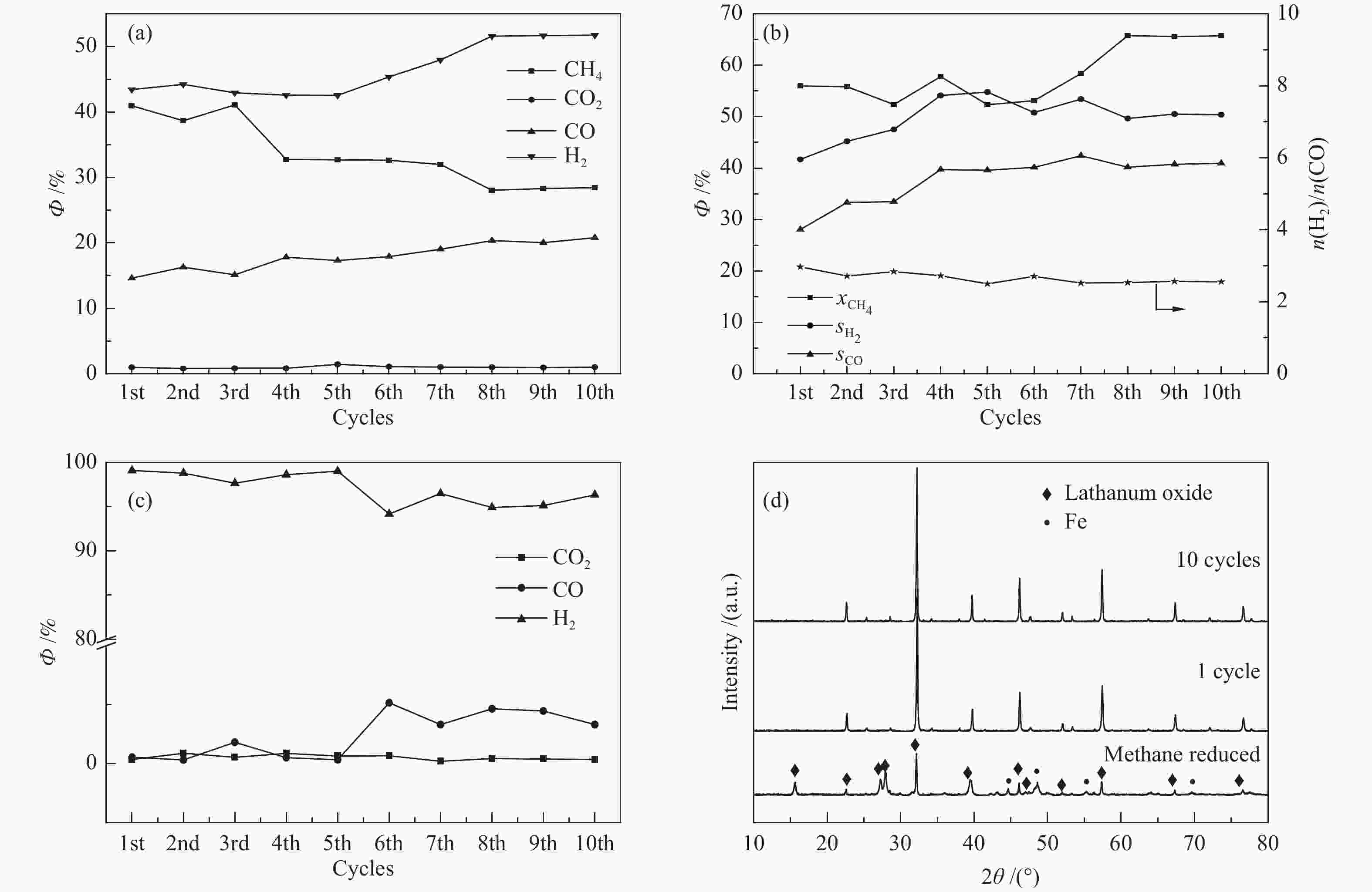
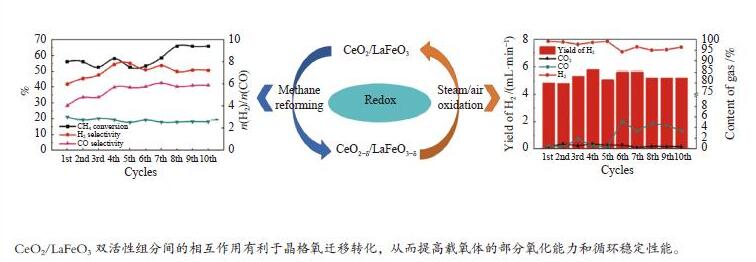
摘要: 甲烷化学链重整是一种利用载氧体(金属氧化物)的部分氧化能力以实现甲烷重整制取合成气的工艺,同时氧化过程中利用水蒸气氧化,还原态的载氧体在恢复晶格氧的同时分解水蒸气制氢。利用溶胶-凝胶法制备载氧体CeO2/LaFeO3,通过X射线粉末衍射和程序升温还原等材料表征方法分析该载氧体的结构特点以及供氧能力,借助于固定床反应实验探讨了组分比例、反应温度对该载氧体反应性能的影响。实验结果表明,CeO2的含量对该载氧体的供氧能力有着显著影响,合适的反应温度不仅有利于甲烷活化,而且能够促进载氧体中晶格氧的迁移,从而提高载氧体的选择性。当CeO2的添加量为10%,反应温度为850 ℃时,该载氧体的反应性能最优,甲烷转化率可以达到94%,H2选择性和CO选择性分别可以达到90%、83%。在连续的氧化-还原循环中保持稳定的反应性能和结构。Abstract: Chemical looping methane reforming is a potential route to co-produce syngas and hydrogen by using the oxygen carrier (metal oxide). The oxygen carrier CeO2/LaFeO3 was prepared by sol-gel method, and the structure and oxygen supply capacity of the oxygen carrier were analyzed by X-ray powder diffraction and hydrogen temperature programmed reduction. The influence of CeO2 ratio and reaction temperature on the performance of the oxygen carrier were discussed through fixed bed reaction tests. The content of CeO2 had a significant effect on the oxygen supply capacity of the oxygen carrier. Increasing reaction temperature not only was conducive to methane activation, but also enhanced lattice oxygen migration in the oxygen carrier. A suitable reaction temperature could match methane activation with lattice oxygen migration, thereby improving the selectivity of the oxygen carrier. Experimental results showed that performance of the oxygen carrier was in the optimal when CeO2 content was 10% and reaction temperature was 850 ºC. CH4 conversion rate could reach 94%, H2 selectivity and CO selectivity could reach 90% and 83%, respectively. The oxygen carrier 10%CeO2/LaFeO3 could maintain stable reaction performance and structure in the redox cycles.
-
Key words:
- methane /
- chemical looping reforming /
- syngas /
- oxygen carrier /
- selectivity
-
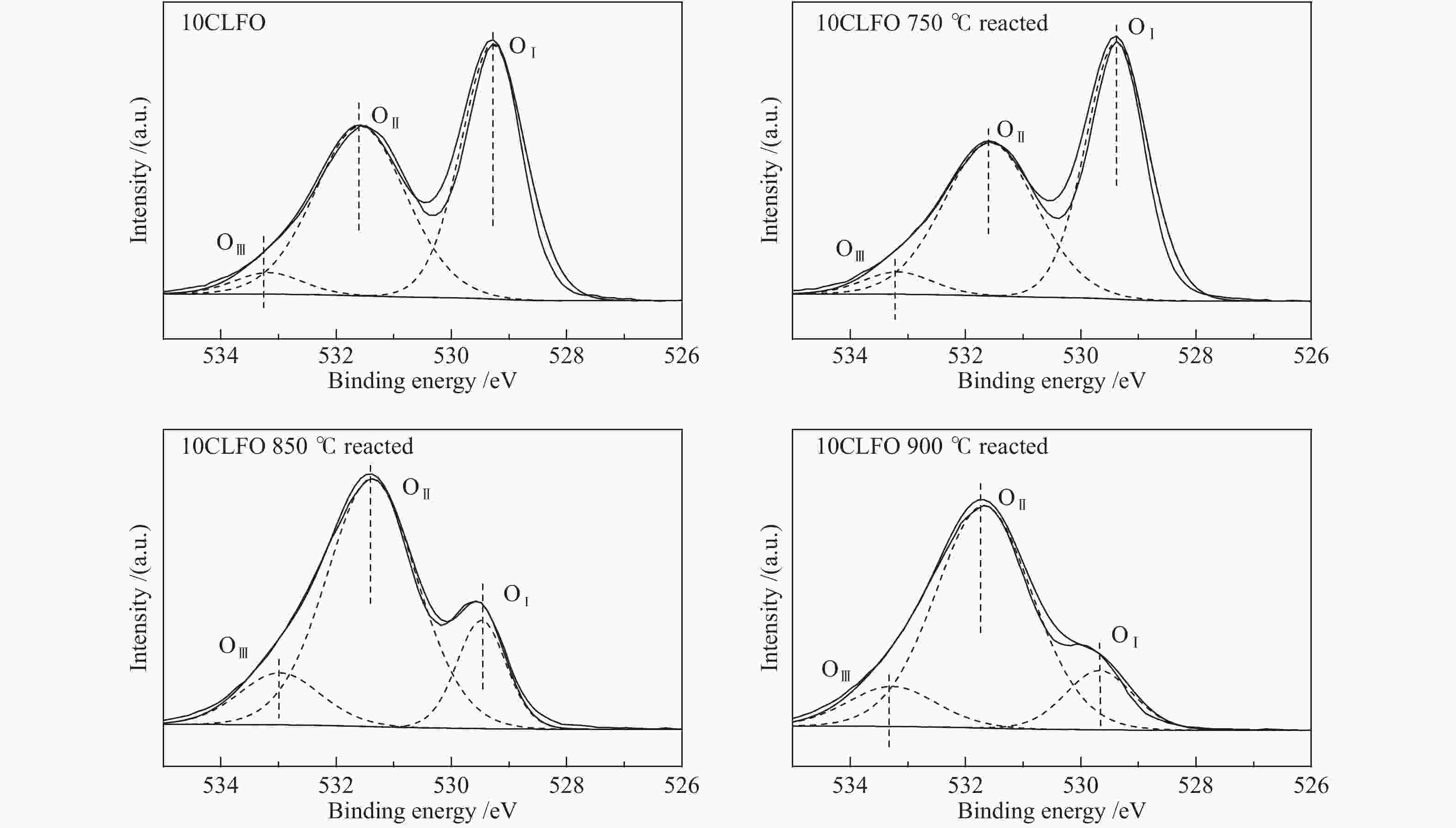
表 1 氧物种相对含量
Table 1 Contents of different oxygen species
Sample OI/% OII/% OIII/% OLat /Oads 10CLFO 49.05 46.55 4.40 0.96 10CLFO-750 50.44 44.7 4.86 1.02 10CLFO-850 16.65 70.13 13.22 0.20 10CLFO-900 13.79 74.02 12.19 0.16 -
[1] 沈阳, 赵坤, 何方, 李海滨. 三维有序大孔钙钛矿型氧化物LaFe0.7Co0.3O3的合成及甲烷化学链水蒸气重整性能[J]. 燃料化学学报,2016,44(10):1168−1176. doi: 10.3969/j.issn.0253-2409.2016.10.003SHEN Yang, ZHAO Kun, HE Fang, LI Hai-bin. Synthesis of three-dimensionally ordered macroporous LaFe0.7Co0.3O3 perovskites and their performance for chemical- looping steam reforming of methane[J]. J Fuel Chem Technol,2016,44(10):1168−1176. doi: 10.3969/j.issn.0253-2409.2016.10.003 [2] RICHTER H J, KNOCHE K F. Reversibility of combustion process, efficiency and costing, second law analysis processes[J]. ACS Symp Ser,1983,235:71−85. [3] RYDÉN M, LYNGFELT A, MATTISSON T. Synthesis gas generation by chemical-looping reforming in a continuously operating laboratory reactor[J]. Fuel,2006,85(12/13):1631−1641. doi: 10.1016/j.fuel.2006.02.004 [4] CABELLO A, ABAD A, GARCÍA-LABIANO F, GAYÁN P, DE DIEGO L F, ADÁNEZ J. Kinetic determination of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier for use in gas-fueled chemical looping combustion[J]. Chem Eng J,2014,258:265−280. doi: 10.1016/j.cej.2014.07.083 [5] JUAN A, ALBERTO A, FRANCISCO G L, PILAR G, LUIS F. D D. Progress in chemical-looping combustion and reforming technologies[J]. Prog Energy Combust,2012,38(2):215−282. doi: 10.1016/j.pecs.2011.09.001 [6] JOHANSSON M, MATTISSON T, LYNGFELT A. Investigation of Mn3O4 with stabilized ZrO2 for chemical-looping combustion[J]. Chem Eng Res Des,2006,84(9):807−818. doi: 10.1205/cherd.05206 [7] WANG B W, YAN R, ZHAO H B, ZHENG Y, LIU Z H, ZHENG C G. Investigation of chemical looping combustion of coal with CuFe2O4 oxygen carrier[J]. Energy Fuels,2011,25(7):3344−3354. doi: 10.1021/ef2004078 [8] 徐艳, 堵锡华, 李靖, 王鹏, 朱捷, 葛奉娟, 周俊, 宋明, 朱文友. SiO2和Al2O3负载的Ni基催化剂在甲烷干重整中的催化性能差异[J]. 燃料化学学报,2019,47(2):199−207. doi: 10.3969/j.issn.0253-2409.2019.02.009XU Yan, DU Xi-hua, LI Jing, WANG Peng, ZHU Jie, GE Feng-juan, ZHOU Jun, SONG Ming, ZHU Wen-you. A comparison of Al2O3 and SiO2 supported Ni-based catalysts in their performance for the dry reforming of methane[J]. J Fuel Chem Technol,2019,47(2):199−207. doi: 10.3969/j.issn.0253-2409.2019.02.009 [9] ZHU X, LI K Z, NEAL L, LI F X. Perovskites as geo-inspired oxygen storage materials for chemical looping and three-way catalysis: A perspective[J]. ACS Catal,2018,8(9):8213−8236. doi: 10.1021/acscatal.8b01973 [10] 周则龄, 张萌, 张俊峰, 宋法恩, 张清德, 谭猗生, 韩怡卓. 钙钛矿型氧化物负载 Ni 催化剂上甲烷二氧化碳重整反应研究[J]. 燃料化学学报,2020,48(7):833−840. doi: 10.3969/j.issn.0253-2409.2020.07.008ZHOU Ze-ling, ZHANG Meng, ZHANG Jun-feng, SONG Fa-en, ZHANG Qing-de, TAN Yi-sheng, HAN Yi-zhuo. Methane reforming with carbon dioxide over the perovskite supported Ni catalysts[J]. J Fuel Chem Technol,2020,48(7):833−840. doi: 10.3969/j.issn.0253-2409.2020.07.008 [11] 靳南南, 张立, 朱燕燕, 刘瑞林, 马晓迅, 王晓东. 甲烷化学链重整制合成气用氧载体的研究进展[J]. 天然气化工(C1化学与化工),2019,44(3):106−116. doi: 10.3969/j.issn.1001-9219.2019.03.025JIN Nan-nan, ZHANG Li, ZHU Yan-yan, LIU Rui-lin, MA Xiao-xun, WANG Xiao-dong. Research progresses of oxygen carriers for chemical looping reforming of methane to syngas[J]. Nat Gas Chem Ind,2019,44(3):106−116. doi: 10.3969/j.issn.1001-9219.2019.03.025 [12] DAI X P, LI R J, YU C C, HAO Z P. Unsteady-state direct partial oxidation of methane to synthesis gas in a fixed-bed reactor using AFeO3 (A = La, Nd, Eu) perovskite-type oxides as oxygen storage[J]. J Phys Chem B,2006,110(45):22525−22531. doi: 10.1021/jp063490b [13] MIHAI O, CHEN D, HOLMEN A. Catalytic consequence of oxygen of lanthanum ferrite perovskite in chemical looping reforming of methane[J]. Ind Eng Chem Res,2011,50(5):2613−2621. doi: 10.1021/ie100651d [14] ZHAO K, HE F, HUANG Z, ZHENG A Q, LI H B, ZHAO Z L. Three-dimensionally ordered macroporous LaFeO3 perovskites for chemical-looping steam reforming of methane[J]. Int J Hydrogen Energy,2014,39(7):3243−3252. doi: 10.1016/j.ijhydene.2013.12.046 [15] DAI X P, LI J, FAN J T, WEI W S, XU J. Synthesis gas generation by chemical-looping reforming in a circulating fluidized bed reactor using perovskite LaFeO3-based oxygen carriers[J]. Ind Eng Chem Res,2012,51(34):11072−11082. doi: 10.1021/ie300033e [16] RYDEN M, LYNGFELT A, MATTISSON T, CHEN D, HOLMEN A, BJORGUM E. Novel oxygen-carrier materials for chemical-looping combustion and chemical-looping reforming; LaxSr1−xFeyCo1−yO3−δ perovskites and mixed-metal oxides of NiO, Fe2O3 and Mn3O4[J]. Int J Greenhouse Gas Control,2008,2(1):21−36. doi: 10.1016/S1750-5836(07)00107-7 [17] LI D Y, XU R D, LI X Y, LI Z Q, ZHU X, LI K Z. Chemical looping conversion of gaseous and liquid fuels for chemical production: A review[J]. Energy Fuels,2020,34(5):5381−5413. [18] 苏迎辉, 郑浩, 张磊, 曾亮. LaMn1-x-yFexCoyO3-δ钙钛矿载氧体用于化学链部分氧化[J]. 化工学报,2020,71(11):5265−5277.SU Yin-hui, ZHENG Hao, ZHANG Lei, ZENG Liang. LaMn1-x-yFexCoyO3-δ perovskite based oxygen carriers for chemical looping partial oxidation[J]. CIESC J,2020,71(11):5265−5277. [19] ZHAO K, LI L W, ZHENG A Q, HUANG Z, HE F, SHEN Y, WEI G Q, LI H B, ZAHO Z L. Synergistic improvements in stability and performance of the double perovskite-type oxides La2-xSrxFeCoO6 for chemical looping steam methane reforming[J]. Appl Energy,2017,197:393−404. doi: 10.1016/j.apenergy.2017.04.049 [20] NEAL L M., SHAFIEFARHOOD A A, LI F X. Dynamic methane partial oxidation using a Fe2O3@La0.8Sr0.2FeO3-δ core-shell redox catalyst in the absence of gaseous oxygen[J]. ACS Catal,2014,4(10):3560−3569. doi: 10.1021/cs5008415 [21] CHEN Y G, GALINSKY N, WANG Z R, LI F X. Investigation of perovskite supported composite oxides for chemical looping conversion of syngas[J]. Fuel,2014,134:521−530. doi: 10.1016/j.fuel.2014.06.017 [22] OTSUKA K, USHIYAMA T, YAMANAKA I. Partial oxidation of methane using the redox of cerium oxide[J]. Chem Lett,1993,22(9):1517−1520. doi: 10.1246/cl.1993.1517 [23] ZHENG Y E, LI K Z, WANG H, ZHU X, WEI Y G, ZHENG M, WANG Y H. Enhanced activity of CeO2-ZrO2 solid solutions for chemical-looping reforming of methane via tuning the macroporous structure[J]. Energy Fuels,2016,30(1):638−647. doi: 10.1021/acs.energyfuels.5b02151 [24] ZHU X, WEI Y G, WANG H, LI K Z. Ce-Fe oxygen carriers for chemical-looping steam methane reforming[J]. Int J Hydrogen Energy,2013,38(11):4492−4501. doi: 10.1016/j.ijhydene.2013.01.115 [25] DING H R, LUO C, LI X S, CAO D S, SHEN Q W, ZHANG L Q. Development of BaSrCo-based perovskite for chemical-looping steam methane reforming: A study on synergistic effects of A-site elements and CeO2 support[J]. Fuel,2019,253:311−319. doi: 10.1016/j.fuel.2019.04.150 [26] 齐凯, 谢峻林, 方德, 李凤祥, 何峰. N2气氛下焙烧制备的Mn基催化剂催化NOx脱除性能的提升机理: 低MnOx结晶度与氧化度(英文)[J]. 催化学报,2017,38(5):845−852. doi: 10.1016/S1872-2067(17)62814-6QI Kai, XIE Jun-Lin, FANG De, LI Feng-xiang, HE Feng. Performance enhancement mechanism of Mn-based catalysts prepared under N2 for NOx removal: Evidence of the poor crystallization and oxidation of MnOx[J]. Chin J Catal,2017,38(5):845−852. doi: 10.1016/S1872-2067(17)62814-6 [27] 李孔斋, 王华, 魏永刚, 敖先权, 刘明春. 晶格氧部分氧化甲烷制合成气[J]. 化学进展,2008,20(9):1306−1314.LI Kong-zhai, WANG Hua, WEI Yong-gang, AO Xian-quan, LIU Ming-chun. Partial oxidation of methane to synthesis gas using lattice oxygen[J]. Prog Chem,2008,20(9):1306−1314. [28] CRLEY A F, ROBERTS M W, SANTRA A K. Interaction of oxygen and carbon monoxide with CsAu surfaces[J]. J Phys Chem B,1997,101(48):9978−9983. doi: 10.1021/jp971780+ [29] ZHANG R D, VILLANUEVA A, ALAMDARI H, KALIAGUINE S. Cu- and Pd-substituted nanoscale Fe-based perovskites for selective catalytic reduction of NO by propene[J]. J Catal,2006,237(2):368−380. doi: 10.1016/j.jcat.2005.11.019 [30] 陈定凯, 张德华, 何德东, 路继长, 钟丽萍, 韩彩云, 罗永明. 杂原子(Zr, Y)掺杂的铈基催化剂中氧物种与其催化CH3SH分解的活性/稳定性之间的关系[J]. 催化学报,2018,39(12):1929−1941. doi: 10.1016/S1872-2067(18)63146-8CHEN Ding-kai, ZHANG De-hua, HE De-dong, LU Ji-chang, ZHONG Li-ping, HAN Cai-yun, LUO Yong-ming. Relationship between oxygen species and activity/stability in heteroatom (Zr, Y) -doped cerium-based catalysts for catalytic decomposition of CH3SH[J]. Chin J Catal,2018,39(12):1929−1941. doi: 10.1016/S1872-2067(18)63146-8 [31] MIHAI O, CHEN D, HOLMEN A. Chemical looping methane partial oxidation: The effect of the crystal size and O content of LaFeO 3[J]. J Catal,2012,293:175−185. doi: 10.1016/j.jcat.2012.06.022 [32] LI K Z, WANG H, WEI Y G, YAN D X. Syngas production from methane and air via a redox process using Ce-Fe mixed oxides as oxygen carriers[J]. Appl Catal B: Environ,2010,97(3/4):361−372. [33] 李琳, 闪洁, 杨桢, 张煜华, 李金林. 催化剂 Ni-CeO2的制备及其在甲烷二氧化碳重整反应中的催化性能[J]. 中南民族大学学报,2018,37(4):1−6.LI Ling, SHAN Jie, YANG Zhen, ZHANG Yu-hua, LI Jin-lin. Preparation of Ni-CeO2 and its catalytic performance in carbon dioxide reforming of methane[J]. J South-Cent Univ Natl,2018,37(4):1−6. [34] LI K Z, WANG H, WEI Y G, LIU M C. Catalytic performance of cerium iron complex oxides for partial oxidation of methane to synthesis gas[J]. J Rare Earth,2008,26(5):705−710. doi: 10.1016/S1002-0721(08)60167-2 [35] 李孔斋, 王华, 魏永刚, 刘明春. 铈钴复合氧化物催化甲烷裂解制氢及两步法制合成气实验研究[J]. 中国稀土学报,2008,26(2):129−134.LI Kong-zhai, WANG Hua, WEI Yong-gang, LIU Ming-chun. Hydrogen production via direct cracking of methane and two step method for syngas over cerium cobalt complex oxides catalyst[J]. J Chin Rare Earth Soc,2008,26(2):129−134. [36] CIAMBELLI P, CIMINO S, LISI L, FATICANTI M, MINELLI G, PETTITI I, PORTA P. La, Ca and Fe oxide perovskites: preparation, characterization and catalytic properties for methane combustion[J]. Appl Catal B: Environ,2001,33(3):193−203. doi: 10.1016/S0926-3373(01)00163-1 [37] ZHANG R, ALAMDARI H, KALIAGUINE S. Fe-based perovskites substituted by copper and palladium for NO+CO reaction[J]. J Catal,2006,242(2):241−253. doi: 10.1016/j.jcat.2006.05.033 [38] THIRUMALAIRAJAN S, GIRIJA K, HEBALKAR N Y, MANGALARAJ D, VISWANATHAN C, PONPANDIAN N. Shape evolution of perovskite LaFeO3 nanostructures: A systematic investigation of growth mechanism, properties and morphology dependent photocatalytic activities[J]. RSC Adv,2013,3:7549−7561. doi: 10.1039/c3ra00006k -




 下载:
下载: