Mechanism of hydrogenation of CO to aromatics from coal pyrolysis gas of low rank under methanol atmosphere
-
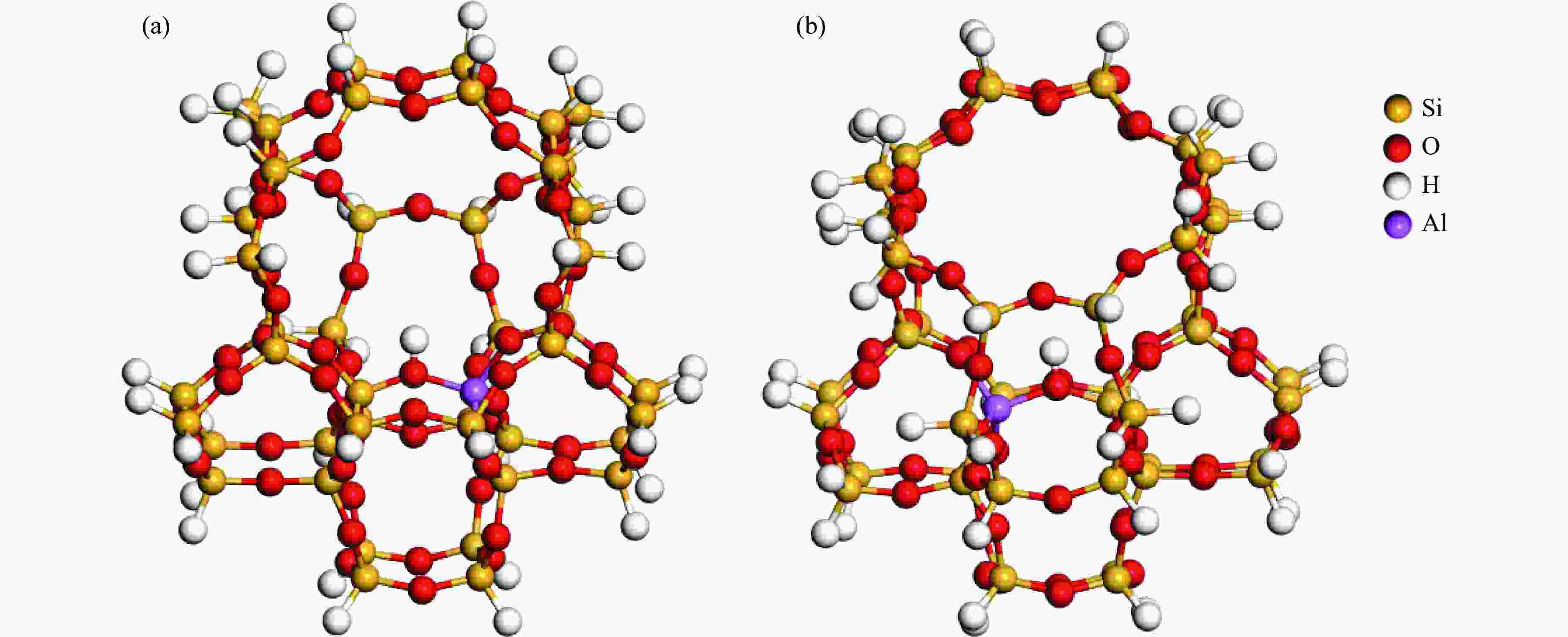
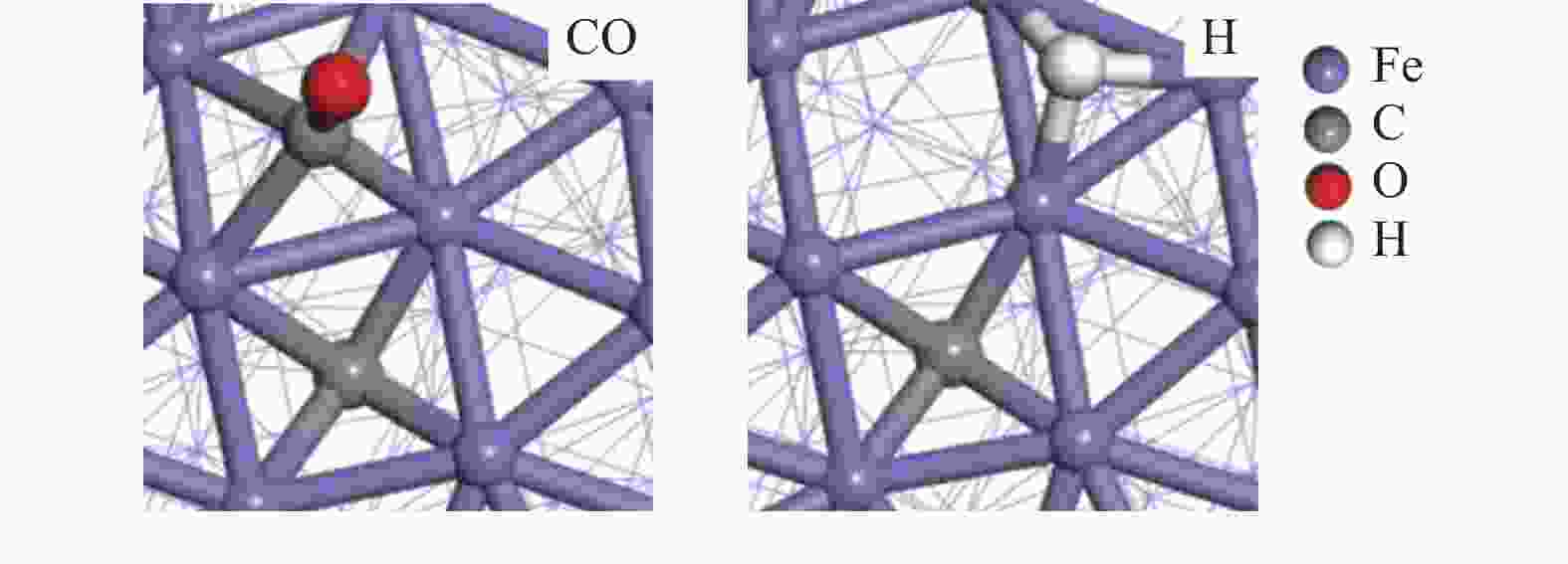
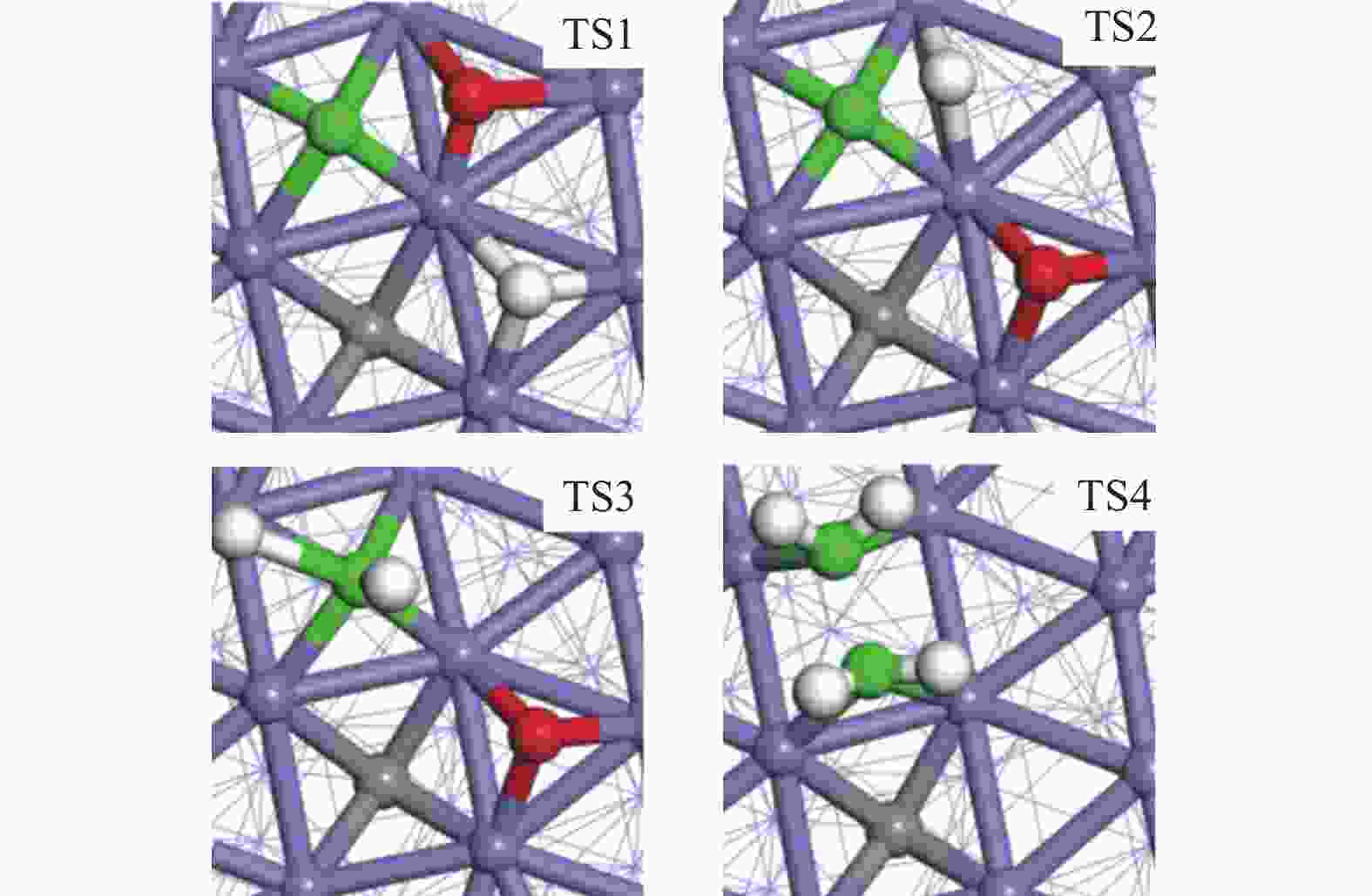
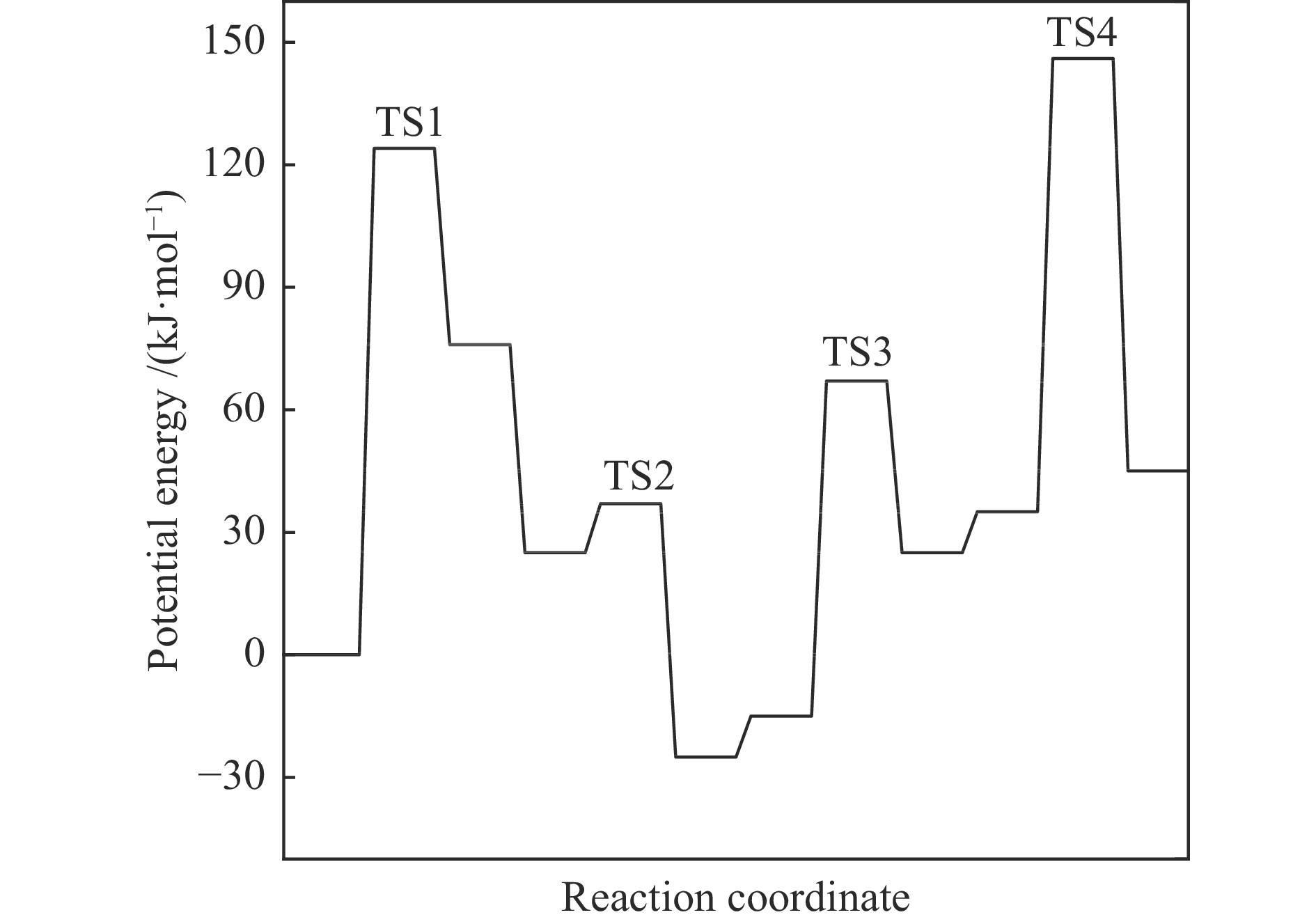
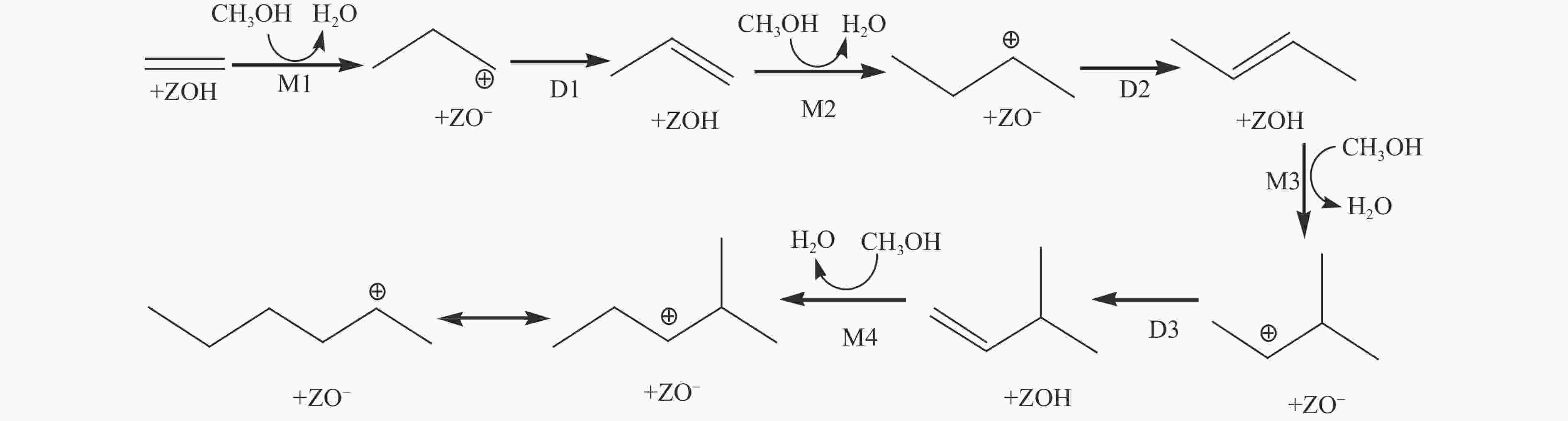
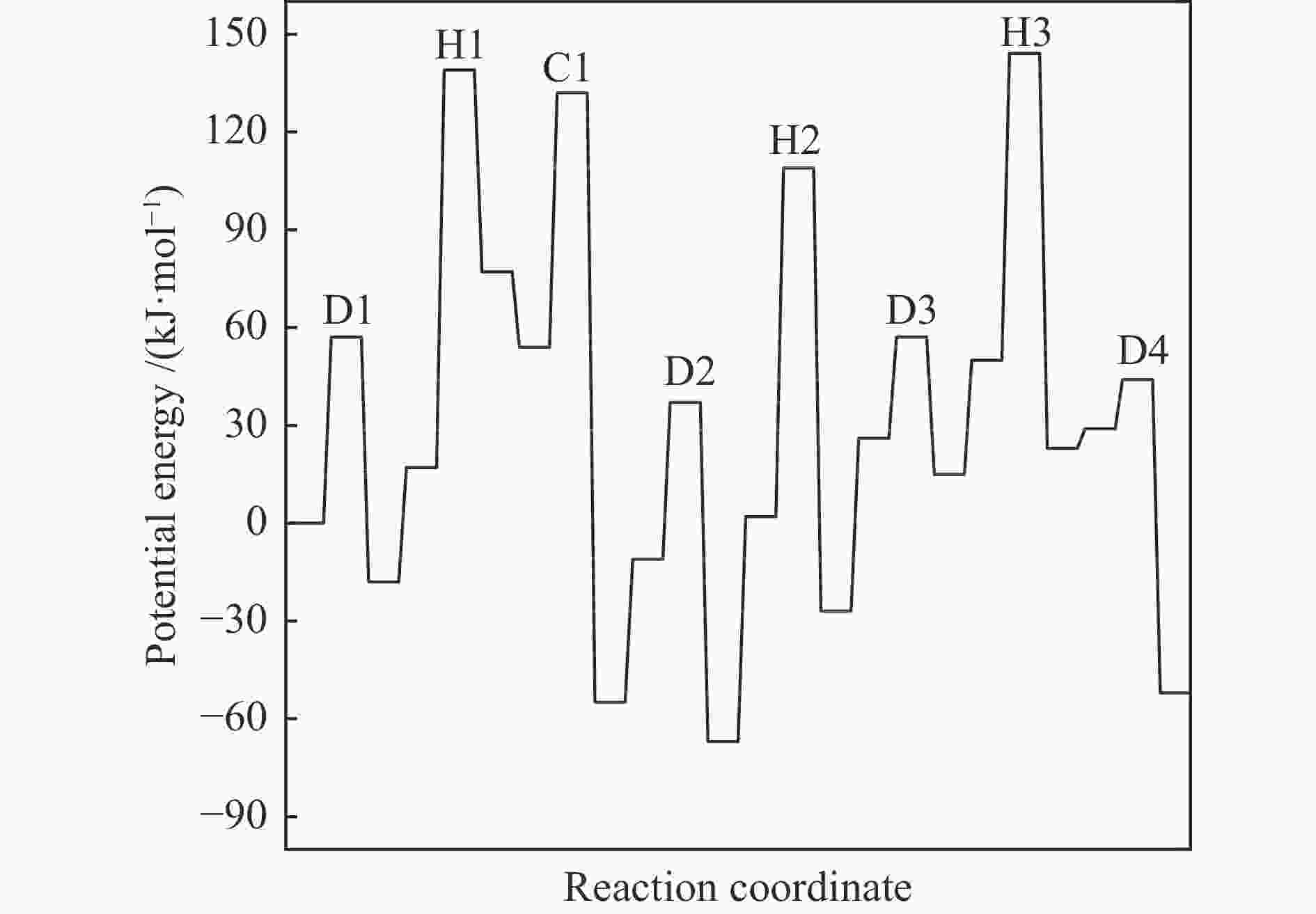
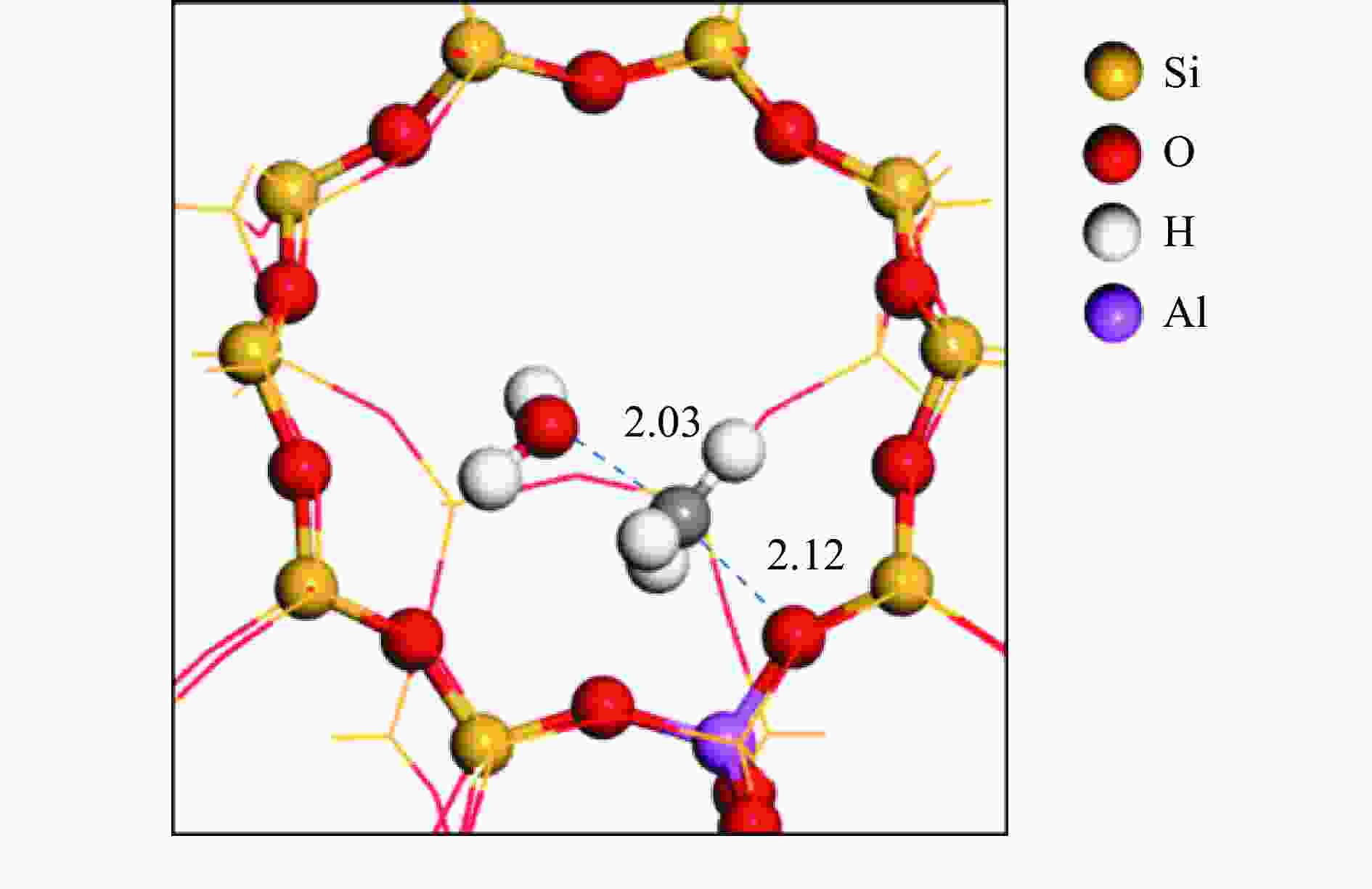
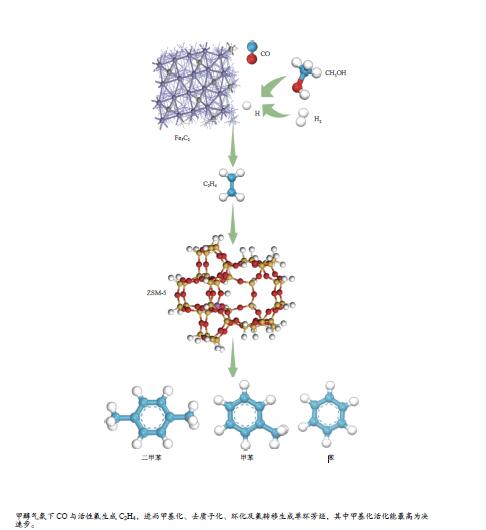
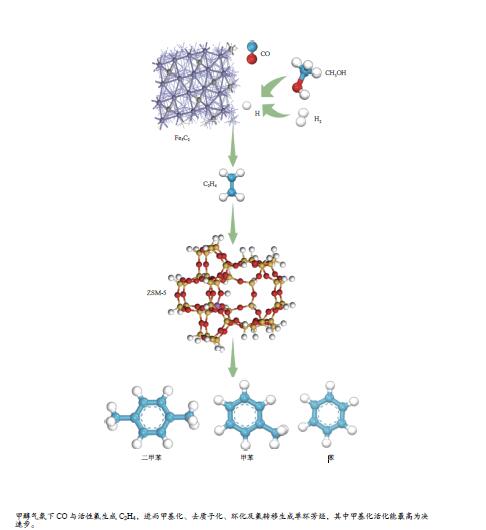
摘要: 由于低阶煤含氧官能团较多,热解过程产生大量CO和CO2,甲醇气氛提供的活性氢可实现CO或CO2催化加氢生成轻质芳烃。本研究采用密度泛函理论探讨了甲醇气氛下低阶煤热解气之一CO于Fe/HZSM-5催化剂上经烯烃中间体制芳烃的机理,结果表明,CO于Fe5C2(510)表面加氢生成低碳烯烃,进而通过多次甲基化和去质子化实现C−C键偶联及链增长,其中,甲基化需活化能较高。
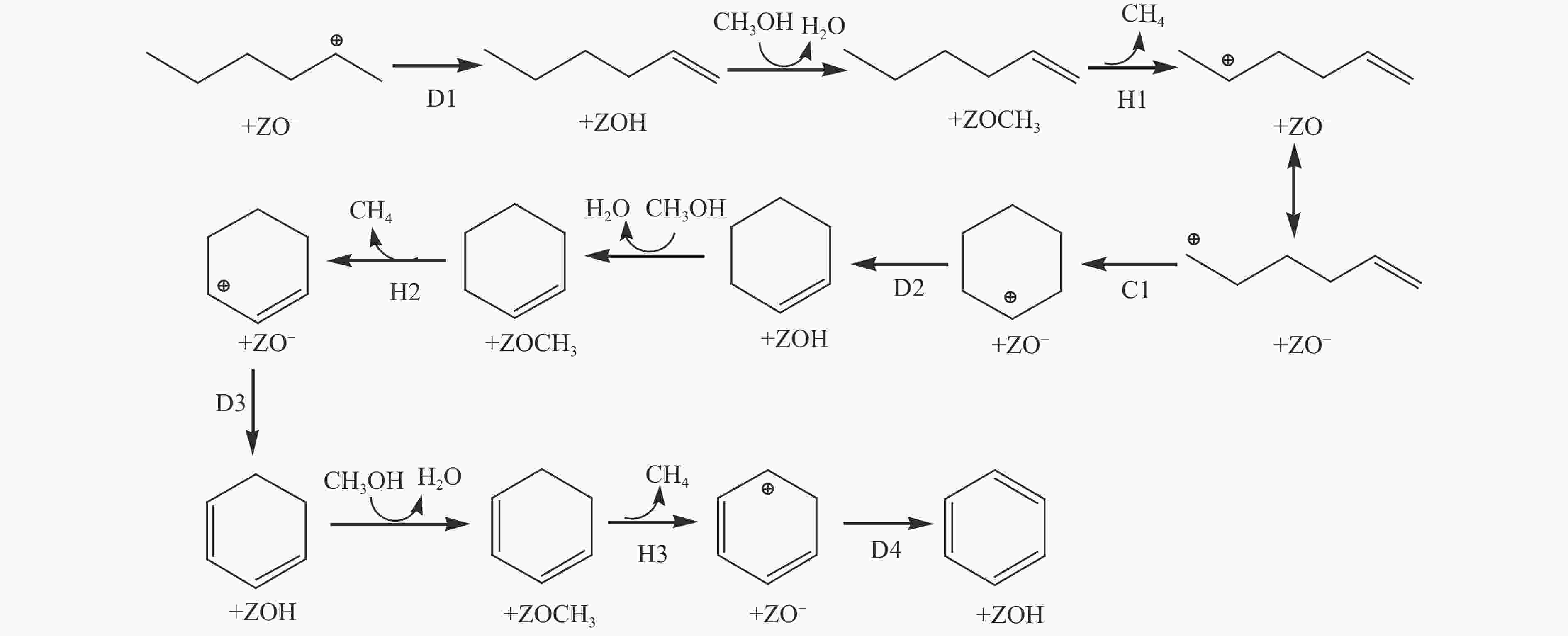
${\rm{C}}^+_{6} $ 芳构化过程通过氢转移、去质子化及环化生成苯,其氢转移最难。整个CO加氢制芳烃过程以甲基化所需能垒最高,成为该反应的决速步。Abstract: Because there were much more oxygen-containing functional groups for low-rank coal, a large amount of CO and CO2 were produced during the pyrolysis process. Catalytic hydrogenation of CO or CO2 to light aromatics could be realized by supplying active hydrogen from methanol. Density functional theory (DFT) was used to investigate the mechanism of CO hydrogenation to aromatics via olefins intermediates over Fe/HZSM-5 catalyst under methanol atmosphere. The results showed that light olefins were formed by CO hydrogenation on Fe5C2 (510) surface. C−C bond coupling and chain propagation were achieved through multiple processes such as methylation and deprotonation. Methylation required a high activation energy among these processes. The aromatization of${\rm{C}}^+_6 $ to benzene was carried out by reactions of hydrogen transfer, deprotonation and cyclization. Hydrogen transfer was the most difficult to happen. In the whole process of CO hydrogenation to aromatics, the energy barrier of methylation was the highest, which was the rate-determining step.-
Key words:
- CO hydrogenation /
- aromatics /
- density functional theory /
- reaction mechanism
-
表 1 生成C2H4计算结果
Table 1 Calculation results of forming C2H4 (823 K,101 kPa)
Reaction ∆H/(kJ·mol−1) ∆G≠/(kJ·mol−1) k/s−1 H + CO→H + C + O 76 124 2.31 × 105 H + C + O→CH + O −50 12 2.81 × 1012 CH + O + H→CH2 + O 75 82 1.03 × 108 CH2 + CH2→CH2CH2 10 112 1.38 × 106 表 2 生成C
${}^+_6 $ 计算结果Table 2 Calculation results of forming C
${}^+_6 $ (823 K,101 kPa)Reaction ∆H/(kJ·mol−1) ∆G≠/(kJ·mol−1) k/s−1 M1 30 135 4.37 × 104 D1 −42 53 7.25 × 109 M2 34 158 1.50 × 103 D2 −41 79 1.64 × 108 M3 51 96 1.35 × 107 D3 −22 61 2.31 × 109 M4 39 165 6.16 × 102 表 3 C
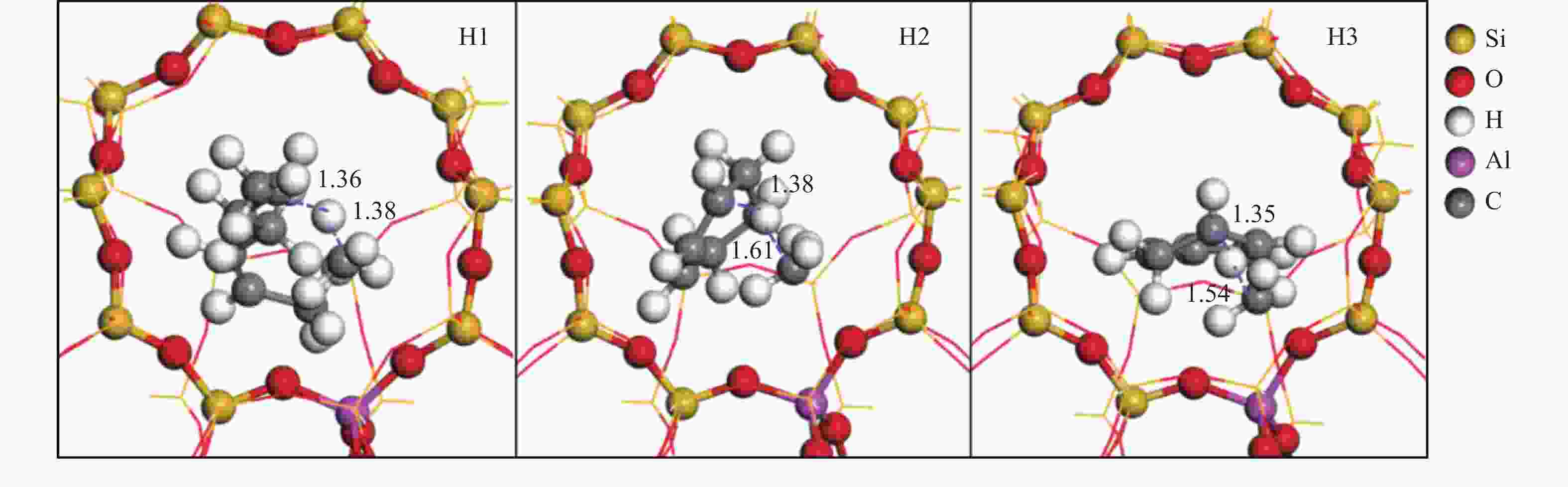
${}^+_6 $ 芳构化计算结果Table 3 Calculation results of C
${}^+_6 $ aromatization (823 K,101 kPa)Reaction ∆H/(kJ·mol−1) ∆G≠/(kJ·mol−1) k/s−1 D1 −18 57 4.13 × 109 H1 60 122 3.10 × 105 C1 −109 78 1.92 × 108 D2 −55 48 1.54 × 1010 H2 −29 107 2.77 × 106 D3 −11 31 1.85 × 1011 H3 −27 94 1.85 × 107 D4 −81 15 1.91 × 1012 -
[1] 赵君强. 煤化工绿色发展研究[J]. 煤炭与化工,2020,43(7):126−127. doi: 10.19286/j.cnki.cci.2020.07.036ZHAO Jun-qiang. Research on green development of coal chemical industry[J]. Coal Chem Ind,2020,43(7):126−127. doi: 10.19286/j.cnki.cci.2020.07.036 [2] 林涛海. 中国煤化工工业发展现况及发展趋向[J]. 化工管理,2021,(19):63−64. doi: 10.19900/j.cnki.ISSN1008-4800.2021.19.028LIN Tao-hai. The present situation and future development trend of coal chemical industry in China[J]. Chem Enterpr Manage,2021,(19):63−64. doi: 10.19900/j.cnki.ISSN1008-4800.2021.19.028 [3] YAN L J, LIU Y J, LV P, WANG M J, LI F, BAO W R. Effect of Brønsted acid of Y zeolite on light arene formation during catalytic upgrading of coal pyrolysis gaseous tar[J]. J Energy Inst,2020,93(6):2247−2254. doi: 10.1016/j.joei.2020.06.007 [4] REN X Y, FENG X B, CAO J P, TANG W, WANG Z H, YANG Z, ZHAO J P, ZHANG L Y, WANG Y J, ZHAO X Y. Catalytic conversion of coal and biomass volatiles: A review[J]. Energy Fuels,2020,34(9):10307−10363. doi: 10.1021/acs.energyfuels.0c01432 [5] HE L, HUI H L, LI S G, LIN W G. Production of light aromatic hydrocarbons by catalytic cracking of coal pyrolysis vapors over natural iron ores[J]. Fuel,2018,216:227−232. [6] LIU Y J, YAN L J, BAI Y H, LI F. Catalytic upgrading of volatile from coal pyrolysis over faujasite zeolites[J]. J Anal Appl Pyrolysis,2018,132:184−189. doi: 10.1016/j.jaap.2018.03.001 [7] LI Y, AMIN M N, LU X M, LI C S, REN F Q, ZHANG S J. Pyrolysis and catalytic upgrading of low-rank coal using a NiO/MgO-Al2O3 catalyst[J]. Chem Eng Sci,2016,155:194−200. doi: 10.1016/j.ces.2016.08.003 [8] KONG X J, BAI Y H, YAN L J, LI F. Catalytic upgrading of coal gaseous tar over Y-type zeolites[J]. Fuel,2016,180:205−210. [9] HE L, LI S G, LIN W G. Catalytic cracking of pyrolytic vapors of low-rank coal over limonite ore[J]. Energy Fuels,2016,30:6984−6990. [10] JIN L J, BAI X Y, LI Y, DONG C , HU H Q, LI X. In-situ catalytic upgrading of coal pyrolysis tar on carbon-based catalyst in a fixed-bed reactor[J]. Fuel Process Technol,2016,147:41−46. [11] XU Y B, YUAN X, CHEN M Y, DONG A L, LIU B, JIANG F, YANG S J, LIU X H. Identification of atomically dispersed Fe-oxo species as new active sites in HZSM-5 for efficient non-oxidative methane dehydroaromatization[J]. J Catal,2021,396:224−241. doi: 10.1016/j.jcat.2021.02.028 [12] CHAREONPANICH M, BOONFUENG T, LIMTRAKUL J. Production of aromatic hydrocarbons from Mae-Moh lignite[J]. Fuel Process Technol,2002,79(2):171−179. doi: 10.1016/S0378-3820(02)00111-X [13] 张海永,王永刚,张培忠,林雄超,朱豫飞. NiW/Al2O3-Y催化剂的制备及其对煤焦油加氢处理的研究[J]. 燃料化学学报,2013,41(9):1085−1091. doi: 10.1016/S1872-5813(13)60046-8ZHANG Hai-yong, WANG Yong-gang, ZHANG Pei-zhong, LIN Xiong-chao, ZHU Yu-fei. Preparation of NiW catalysts with alumina and zeolite Y for hydroprocessing of coal tar[J]. J Fuel Chem Technol,2013,41(9):1085−1091. doi: 10.1016/S1872-5813(13)60046-8 [14] GU Z L, CHANG N, HOU X P, WANG J P, LIU Z K. Experimental study on the coal tar hydrocracking process in supercritical solvents[J]. Fuel,2012,91(1):33−39. doi: 10.1016/j.fuel.2011.07.032 [15] 靳立军, 李扬, 胡浩权. 甲烷活化与煤热解耦合过程提高焦油产率研究进展[J]. 化工学报,2017,68(10):3669−3677. doi: 10.11949/j.issn.0438-1157.20170465JIN Li-jun, LI Yang, HU Hao-quan. Research progress of integrated methane activation with coal pyrolysis for improving coal tar yield[J]. CIESC J,2017,68(10):3669−3677. doi: 10.11949/j.issn.0438-1157.20170465 [16] WANG Y L, YU J P, AN H H, JIN W J, QIAO J Q, SUN Y, CAO J P. Catalytic upgrading of coal tar coupling with methanol using model compound over hierarchal ZSM-5 for increasing light aromatic production under atmosphere pressure[J]. Fuel Process Technol,2021,211:106600. doi: 10.1016/j.fuproc.2020.106600 [17] ZHANG M, MOUTSOGLOU A. Catalytic fast pyrolysis of prairie cordgrass lignin and quantification of products by pyrolysis-gas chromatography-mass spectrometry[J]. Energy Fuels,2014,28(2):1066−1073. doi: 10.1021/ef401795z [18] LI L, FAN H J, HU H Q. A theoretical study on bond dissociation enthalpies of coal based model compounds[J]. Fuel,2015,153:70−77. doi: 10.1016/j.fuel.2015.02.088 [19] FU Y, NI Y M, CHEN Z Y, ZHU W L, LIU Z M. Achieving high conversion of syngas to aromatics[J]. J Eng Chem,2022,66:597−602. [20] XU Y F, WANG J, MA G Y, BAI J Y, DU Y X, DING M Y. Direct synthesis of aromatics from syngas over Mo-modified Fe/HZSM-5 bifunctional catalyst[J]. Appl Catal A: Gen,2020,598:117589. [21] FUJIMOTO K, KUDO Y, TOMINAGA H O, Synthesis gas conversion utilizing mixed catalyst composed of CO reducing catalyst and solid acid:II. Direct synthesis of aromatic hydrocarbons from synthesis gas[J]. J Catal,1984,87(1):136−143. doi: 10.1016/0021-9517(84)90176-3 [22] XU Y, LIU D, LIU X. Conversion of syngas toward aromatics over hybrid Fe-based Fischer-Tropsch catalysts and HZSM-5 zeolites[J]. Appl Catal A: Gen,2018,552:168−183. doi: 10.1016/j.apcata.2018.01.012 [23] CHENG K, ZHOU W, KANG J C, HE S, SHI S l, ZHANG Q H, PAN Y, WEN W, WANG Y. Bifunctional catalysts for one-step conversion of syngas into aromatics with excellent selectivity and stability[J]. Chem,2017,3:334−347. doi: 10.1016/j.chempr.2017.05.007 [24] ZHANG M H, REN J, YU Y Z. Insights into the hydrogen coverage effect and the mechanism of Fischer-Tropsch to olefins process on Fe5C2(510)[J]. ACS Catal,2020,10:689−701. doi: 10.1021/acscatal.9b03639 [25] ZHAO H B, JIANG H, CHENG M, LIN Q, LV Y J, XU Y, XIE J Z, LIU J X, MEN Z W, MA D. Boron adsorption and its effect on stability and CO activation of χ-Fe5C2 catalyst: An ab initio DFT study[J]. Appl Catal A: Gen,2021,627:118382. doi: 10.1016/j.apcata.2021.118382 [26] WANG S, CHEN Y Y, WEI Z H, QIN Z F, MA H, DONG M, LI J F, FAN W B, WANG J G. Polymethylbenzene or alkene cycle? theoretical study on their contribution to the process of methanol to olefins over H-ZSM-5 zeolite[J]. J Phys Chem C,2015,119(51):28482−28498. doi: 10.1021/acs.jpcc.5b10299 -






 下载:
下载: