Photocatalytic mineralization of low concentration phenol facilitated by transfer of positive and negative charges correlation
-
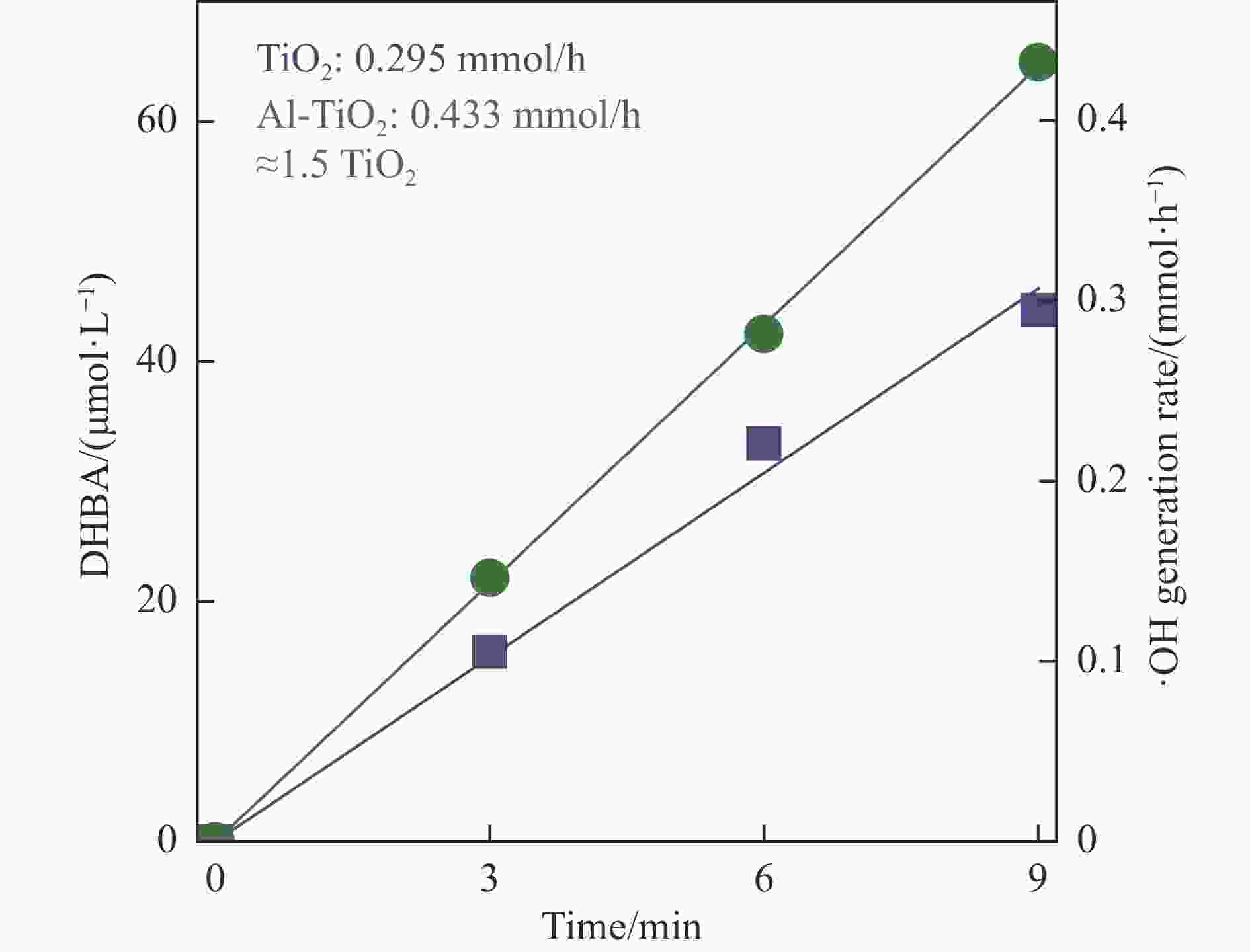
摘要: 光催化矿化难降解污染物(如苯酚)需要羟基自由基(·OH)进行开环反应。本研究通过Al掺杂削弱了TiO2表面对氧物种的吸附,有效促进光致·OH的生成。同时,Al元素还能降低TiO2的导带能级,继而降低半导体-助催化剂界面电子转移势垒而促进还原半反应。由于正负电荷的强关联性,还原半反应中电子的快速转移可提高半导体内空穴浓度,加快·OH的生成。此外,通过将催化剂固定在反应器的光入射内壁,还能避免由污染物竞争光吸收所引起的光子损失。基于这些优点,有望实现废水中低浓度苯酚的高效光催化矿化。Abstract: Photocatalytic mineralization of recalcitrant contaminants such as phenol requires hydroxyl radicals (·OH) for ring-opening reactions. Here, we weaken the adsorption of oxygen species on TiO2 surface by Al doping, which can effectively promote the photoinduced ·OH generation. Besides, Al doping can downshift the conduction band of TiO2. The resulted potential barrier lowering can promote semiconductor-cocatalyst interfacial electron transfer for the reduction half-reaction. Due to the strong correlation between positive and negative charges, the rapid transfer of electrons in the reduction half-reaction can also increase the concentration of holes in the semiconductor and promote the generation of ·OH. By immobilizing the photocatalyst on the light-incident inner wall of the reactor, it can avoid the competitive light absorption by the contaminant. By these virtues, efficient photocatalytic mineralization of low-concentration phenol in wastewater can be realized.
-
Key words:
- phenol /
- photocatalytic /
- mineralization /
- interfacial electron transfer /
- TiO2
-
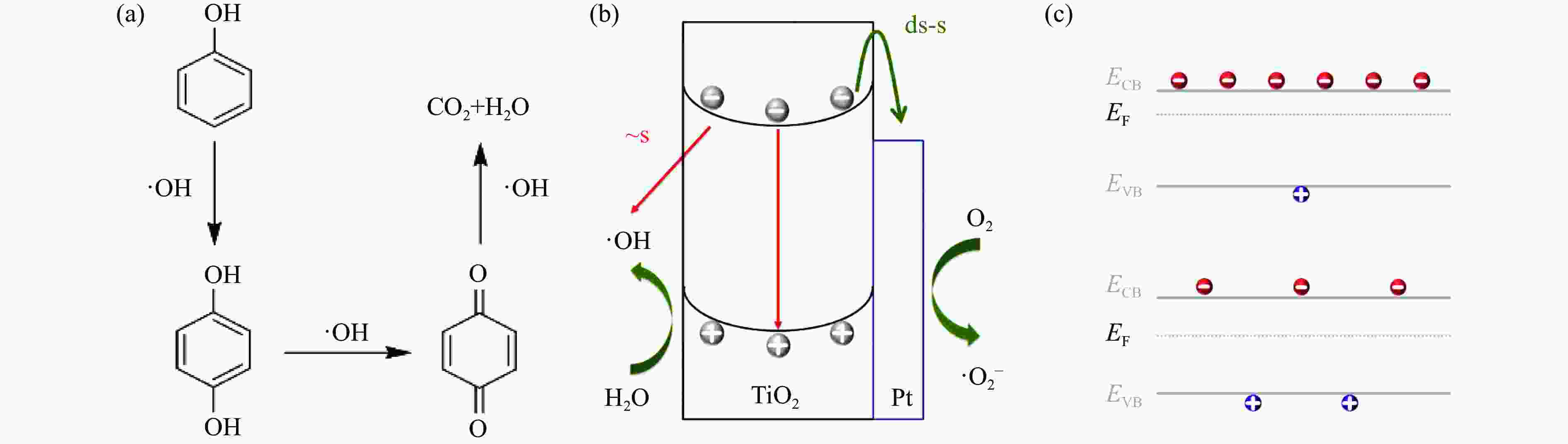
图 1 (a)苯酚开环和矿化示意图[8](改编自Elsevier);(b)光催化反应的基本过程及其时间尺度[10](绿线和红线分别代表正副反应过程);(c)正负电荷内在关联性
Figure 1 (a) Schematic diagram for ring-opening and mineralization of phenol[8] (Adapted from Elsevier); (b) The basic process of photocatalytic reaction and its timescale [10] (The green and red arrows respectively represent the forward and side reactions); (c) Strong correlation between positive and negative charges
图 2 Al掺杂前后的(a)XRD谱图,(b)UV-vis吸收光谱图,(c)TiO2及(d)Al-TiO2的SEM照片,(e)Al-TiO2的高角环形暗场像及元素分析(图中标尺长度为200 nm)
Figure 2 (a) XRD patterns; (b) UV-vis absorption spectra; (c), (d) SEM images of Al-TiO2 and TiO2; (e) the high angle annular dark field image and elemental mappings of Al-TiO2 (the scale bar shown in elemental mappings is 200 nm)
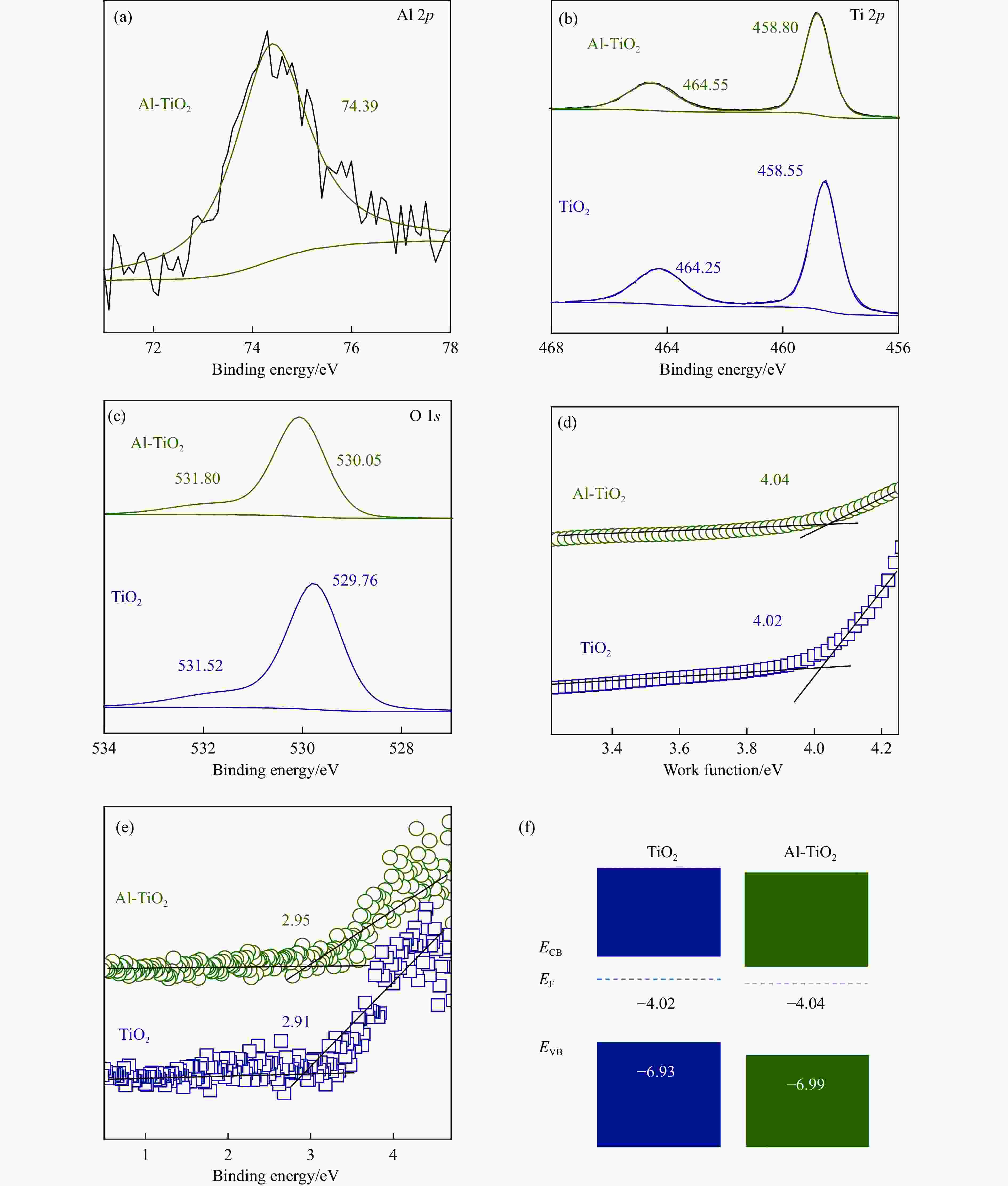
图 3 (a)Al-TiO2的Al 2p XPS谱图;(b)和(c)Al-TiO2和TiO2的Ti 2p和O 1s XPS谱图;(d)由UPS谱图得到的Al-TiO2和TiO2功函数和(e)结合能及(f)能带示意图
Figure 3 (a) Al 2p XPS spectrum of Al-TiO2; (b) Ti 2p and (c) O 1s XPS spectra of Al-TiO2 and TiO2; (d) Work function; (e) Binding energy; (f) The band alignment can be obtained from UPS spectra of Al-TiO2 and TiO2
图 6 Al-TiO2和TiO2在PBS溶液中的(a)水氧化和(b)氧还原反应中的伏安行为曲线
Figure 6 The voltammetry behaviors of Al-TiO2 and TiO2 electrodes in (a) water oxidation and (b) oxygen reduction reaction (For the oxygen reduction reaction, the measurements were conducted when the rotating disk electrode was rotating at a speed of 1600 r/min)
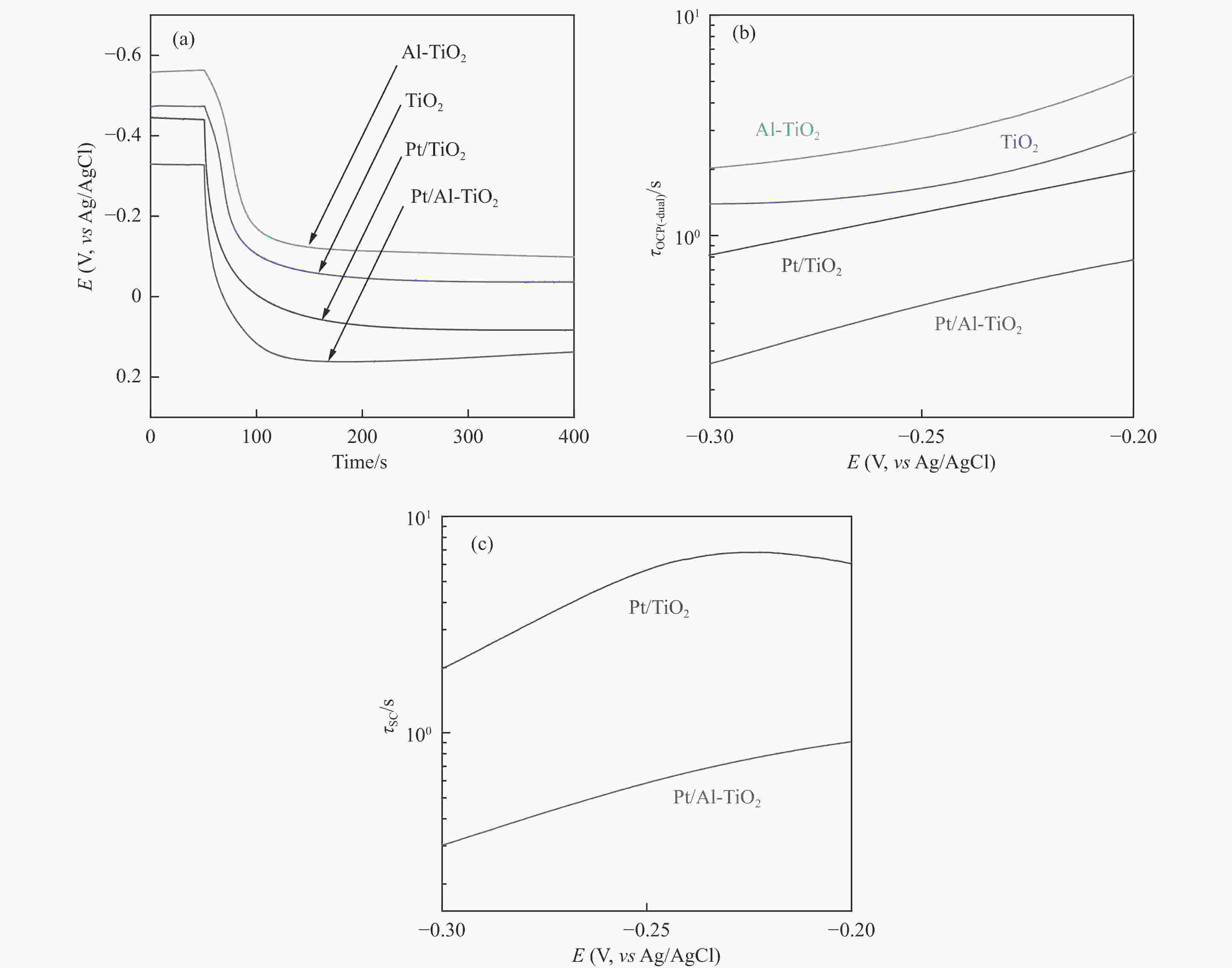
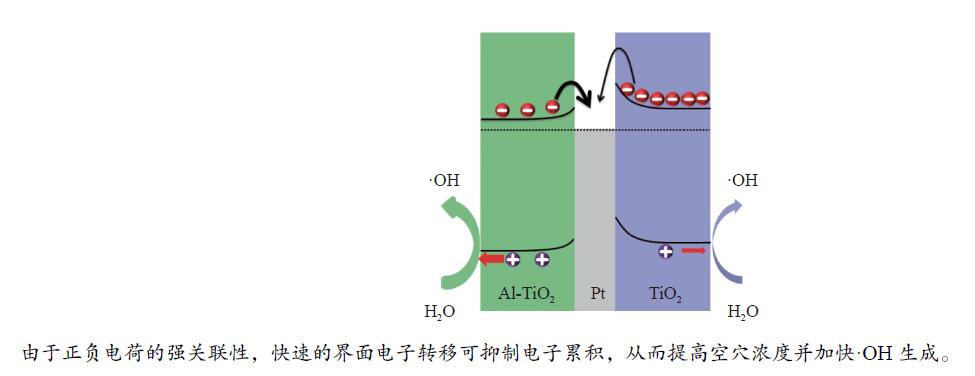
图 7 (a)Al-TiO2、TiO2、Pt/Al-TiO2和Pt/TiO2电极在PBS溶液通氩条件下的OCP行为,以及由此获得的(b)τOCP-(dual)及(c)τSC;(d)导带的下移降低SC界面电子转移势垒高度示意图
Figure 7 (a) OCP behaviors of Al-TiO2, TiO2, Pt/Al-TiO2, and Pt/TiO2 electrodes in argon bubbled PBS solution; The time constants including (b) τOCP-(dual) and (c) τSC can be obtained from the OCP curves; (d) Schematic diagram shows that the downshift of conduction band lowers the potential barrier for SC interfacial electron transfer
图 10 (a)光催化苯酚矿化固定床反应示意图;(b)Pt/Al-TiO2固定床和浆态床反应速率对比;(c)Al-TiO2、TiO2、Pt/Al-TiO2和Pt/TiO2光催化剂对苯酚(COD, 100 mg/L)光催化矿化
Figure 10 (a) Schematic diagram for fixed bed reactor of photocatalytic phenol mineralization; (b) Comparison of Pt/Al-TiO2 reaction rate between fixed bed and slurry suspension; (c) The photocatalytic mineralization of phenol (COD,100 mg/L) over Al-TiO2, TiO2, Pt/Al-TiO2, and Pt/TiO2 photocatalysts
-
[1] JIA K, WEI X H, XU Y, et al. Asymmetric potential barrier lowering promotes photocatalytic nonoxidative dehydrogenation of anhydrous methanol[J]. Appl Catal A: Gen,2023,650:119009−119013. doi: 10.1016/j.apcata.2022.119009 [2] TAKATA T, JIANG J Z, SAKATA Y, et al. Photocatalytic water splitting with a quantum efficiency of almost unity[J]. Nature,2020,581(7809):411−414. doi: 10.1038/s41586-020-2278-9 [3] ALI A, SHOEB M, LI Y, et al. Enhanced photocatalytic degradation of antibiotic drug and dye pollutants by graphene-ordered mesoporous silica (SBA 15)/TiO2 nanocomposite under visible-light irradiation[J]. J Mol Liq,2021,324:114696−114706. doi: 10.1016/j.molliq.2020.114696 [4] DE S FURTADO R X, SABATINI C A, ZAIAT M, et al. Perfluorooctane sulfonic acid (PFOS) degradation by optimized heterogeneous photocatalysis (TiO2/UV) using the response surface methodology (RSM)[J]. J Water Process Eng, 2021, 41: 101986–101993. [5] PASINI S M, VALÉRIO A, YIN G L, et al. An overview on nanostructured TiO2-containing fibers for photocatalytic degradation of organic pollutants in wastewater treatment[J]. J Water Process Eng, 2021, 40: 101827–101842. [6] 彭书传, 王诗生, 陈天虎, 等. 负载型TiO2光催化氧化法处理硝基苯酚工业废水[J]. 工业水处理,2003,23(11):34−36.PENG Shuchuan, WANG Shisheng, CHEN Tianhu, et al. Treatment of nitrophenol wastewater by heterogeneous photocatalytic oxidation process[J]. Ind Water Treat,2003,23(11):34−36. [7] 王九思, 赵红花, 宋光顺, 等. 负载型TiO2固定相光催化降解含酚废水的试验研究[J]. 环境污染治理技术与设备[J],2004,5(10):26−29.WANG Jiu-si, ZHAO Hong-hua, SONG Guang-shun, MA Yan-fei. Experimental research on photocatalytic degradation phenolic wastewater by immobilized TiO2 fixed phase[J]. Tech Equip Environ Pollut Control,2004,5(10):26−29. [8] CHANDANA L, SUBRAHMANYAM C. Degradation and mineralization of aqueous phenol by an atmospheric pressure catalytic plasma reactor[J]. J Environ Chem Eng,2018,6(3):3780−3786. doi: 10.1016/j.jece.2016.11.014 [9] LUO T, WANG Z J, WEI X H, et al. Surface enrichment promotes the decomposition of benzene from air[J]. Catal Sci Technol,2022,12(7):2340−2345. doi: 10.1039/D1CY02296B [10] XIANG H K, WANG Z J, CHEN J Z. Revealing and facilitating the rate-determining step for efficient sunlight-driven photocatalysis[J]. J Phys Chem Lett,2021,12(32):7665−7670. doi: 10.1021/acs.jpclett.1c02101 [11] XIANG H K, WANG Z J, CHEN J Z. Revealing the role of elementary doping in photocatalytic phenol mineralization[J]. Chin J Struc Chem,2022,41(9):2209069−2209073. [12] SARENTUYA, BAI H D, AMURISHANA. Synthesis of Bi2S3-TiO2 nanocomposite and its electrochemical and enhanced photocatalytic properties for phenol degradation[J]. Int J Electrochem Sci,2023,18(4):100071−100079. doi: 10.1016/j.ijoes.2023.100071 [13] HASANI H, SABAHI J, GHAFRI B, et al. Effect of water quality in photocatalytic degradation of phenol using zinc oxide nanorods under visible light irradiation[J]. J Water Process Eng,2022,49:103121−103133. doi: 10.1016/j.jwpe.2022.103121 [14] NOSAKA Y, NOSAKA A Y. Generation and detection of reactive oxygen species in photocatalysis[J]. Chem Rev,2017,117(17):11302−11336. doi: 10.1021/acs.chemrev.7b00161 [15] TAO H B, FANG L W, CHEN J Z, et al. Identification of surface reactivity descriptor for transition metal oxides in oxygen evolution reaction[J]. J Am Chem Soc,2016,138(31):9978−9985. doi: 10.1021/jacs.6b05398 [16] FRIEDMANN D, MENDIVE C, BAHNEMANN D. TiO2 for water treatment: Parameters affecting the kinetics and mechanisms of photocatalysis[J]. Appl Catal B: Environ, 2010, 99(3/4): 398–406. [17] HOFFMANN M R, MARTIN S T, CHOI W Y, et al. Environmental applications of semiconductor photocatalysis[J]. Chem Rev,1995,95(1):69−96. doi: 10.1021/cr00033a004 [18] SCHNEIDER J, MATSUOKA M, TAKEUCHI M, et al. Understanding TiO2 photocatalysis: mechanisms and materials[J]. Chem Rev,2014,114(19):9919−9986. doi: 10.1021/cr5001892 [19] WANG Z J, MEI B B, CHEN J Z. Removing semiconductor–cocatalyst interfacial electron transfer induced bottleneck for efficient photocatalysis: A case study on Pt/CdS photocatalyst[J]. J Catal,2022,408:270−278. doi: 10.1016/j.jcat.2022.03.014 [20] XU Y, WANG Z J, XIANG H K, et al. Revealing the role of electronic doping for developing cocatalyst–free semiconducting photocatalysts[J]. J Phys Chem Lett,2022,13(8):2039−2045. doi: 10.1021/acs.jpclett.2c00193 [21] YANG D L, WANG Z J, CHEN J Z. Revealing the role of surface elementary doping in photocatalysis[J]. Catal Sci Technol,2022,12(11):3634−3638. doi: 10.1039/D2CY00410K [22] 刘诺, 钟志亲, 张桂平, 等. 半导体物理导论[M]. 北京: 科学出版社, 2014: 86–87.LIU Nuo, ZHONG Zhiqin, ZHANG Guiping, CHEN Jinju. Introduction to Semiconductor Physics[M]. Beijing: Science Press, 2014: 86–87. [23] WEI X H, LIU H F, GAO S G, et al. Photocatalyst: to be dispersed or to be immobilized? The crucial role of electron transport in photocatalytic fixed bed reaction[J]. J Phys Chem Lett,2022,13(41):9642−9648. doi: 10.1021/acs.jpclett.2c02581 [24] WU T Z, SUN S N, SONG J J, et al. Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation[J]. Nat Catal,2019,2(9):763−772. doi: 10.1038/s41929-019-0325-4 [25] WANG H Y, HUNG S F, CHEN H Y, et al. In operando identification of geometrical-site-dependent water oxidation activity of spinel Co3O4[J]. J Am Chem Soc,2016,138(1):36−39. doi: 10.1021/jacs.5b10525 [26] HOEX B, HEIL S B S, LANGEREIS E, et al. Ultralow surface recombination of c-Si substrates passivated by plasma-assisted atomic layer deposited Al2O3[J]. Appl Phys Lett,2006,89(4):042112−042114. doi: 10.1063/1.2240736 [27] KAUSHIK R, SAMAL P K, HALDER A. Degradation of fluoroquinolone-based pollutants and bacterial inactivation by visible-light-active aluminum-doped TiO2 nanoflakes[J]. ACS Appl Nano Mater,2019,2(12):7898−7909. doi: 10.1021/acsanm.9b01913 [28] MURASHKINA A A, MURZIN P D, RUDAKOVA A V, et al. EMELINE A V, BAHNEMANN D W. Influence of the dopant concentration on the photocatalytic activity: Al–doped TiO2[J]. J Phys Chem C,2015,119(44):24695−24703. doi: 10.1021/acs.jpcc.5b06252 [29] FLOYD R A, WATSON J J, WONG P K. Sensitive assay of hydroxyl free-radical formation utilizing high-pressure liquid-chromatography with electrochemical detection of phenol and salicylate hydroxylation products[J]. J Biochem Biophys Methods, 1984, 10(3/4): 221–235. -




 下载:
下载: