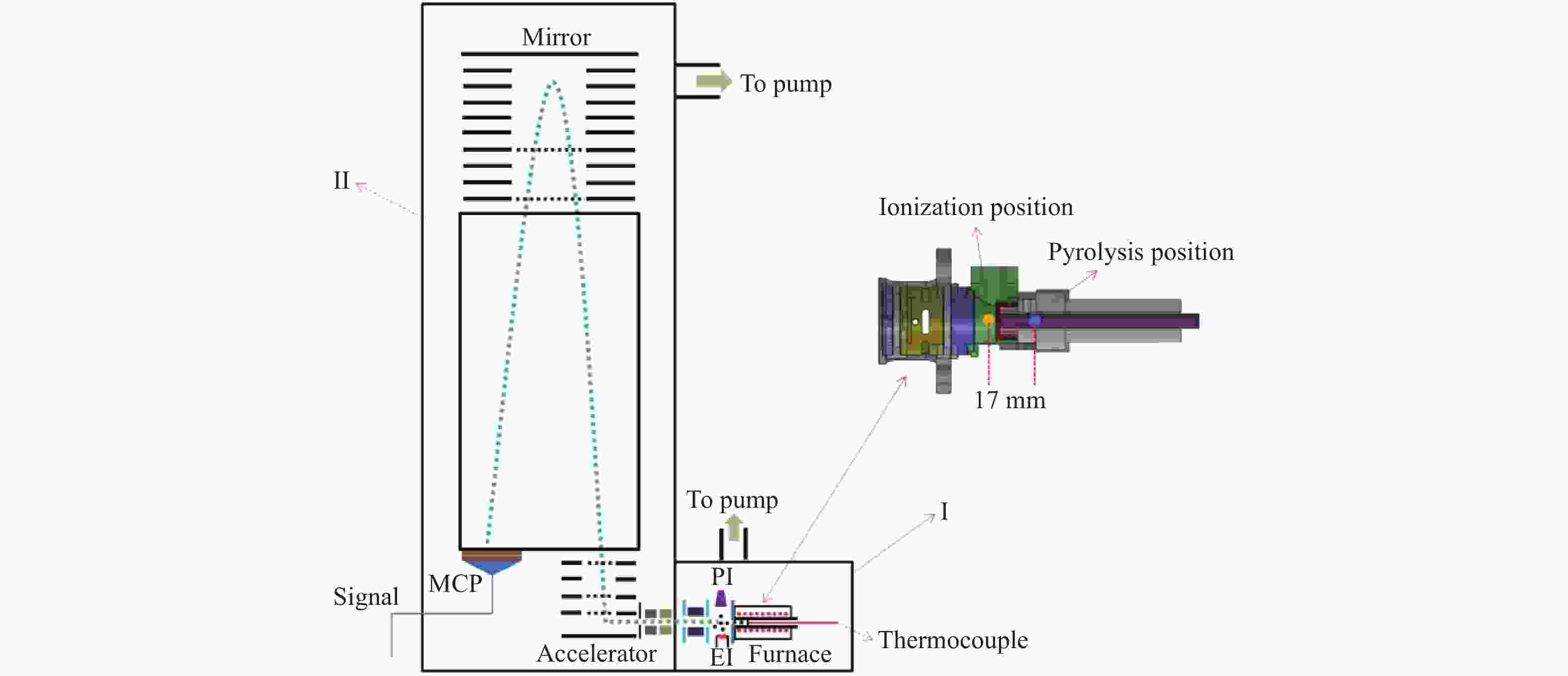
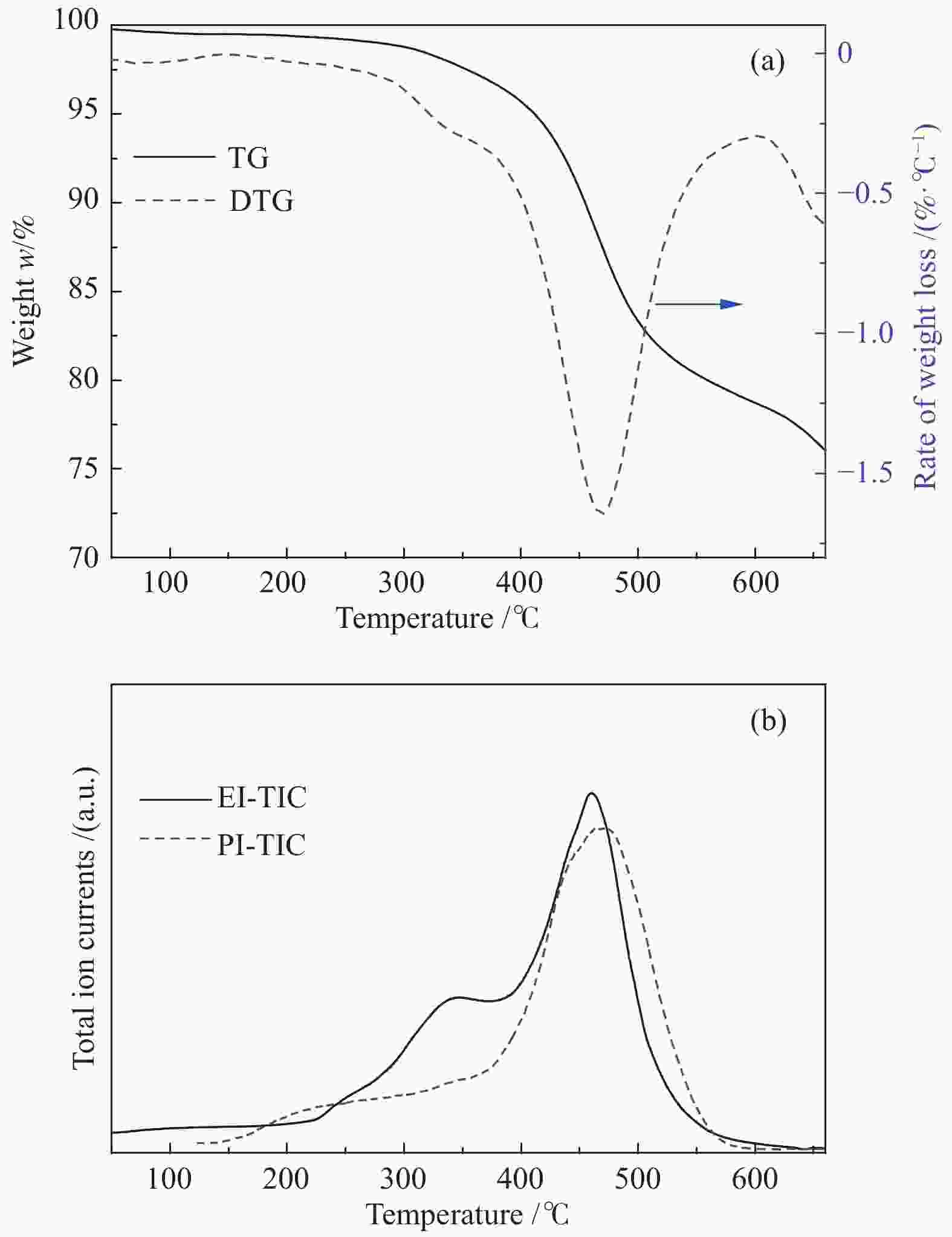
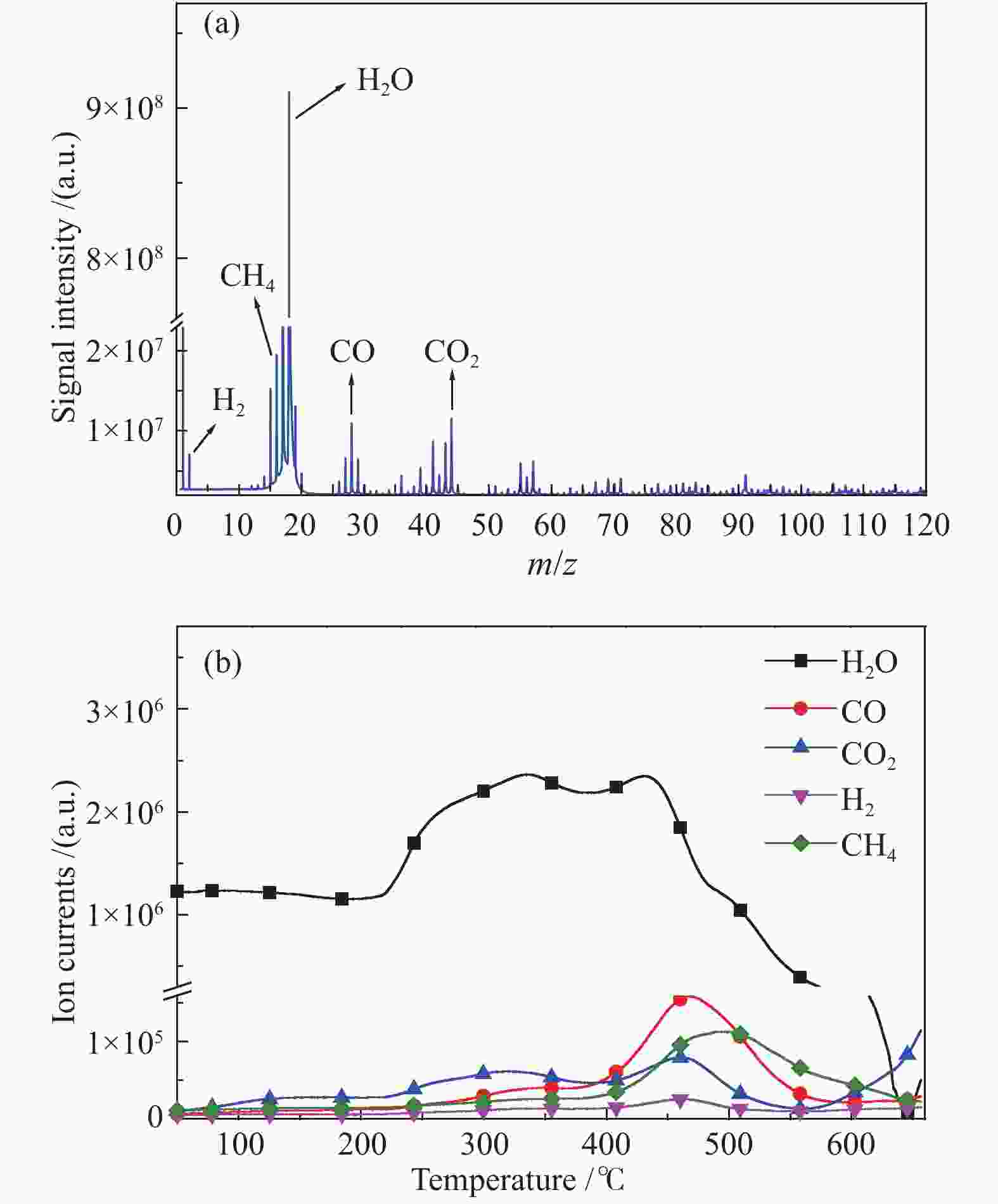
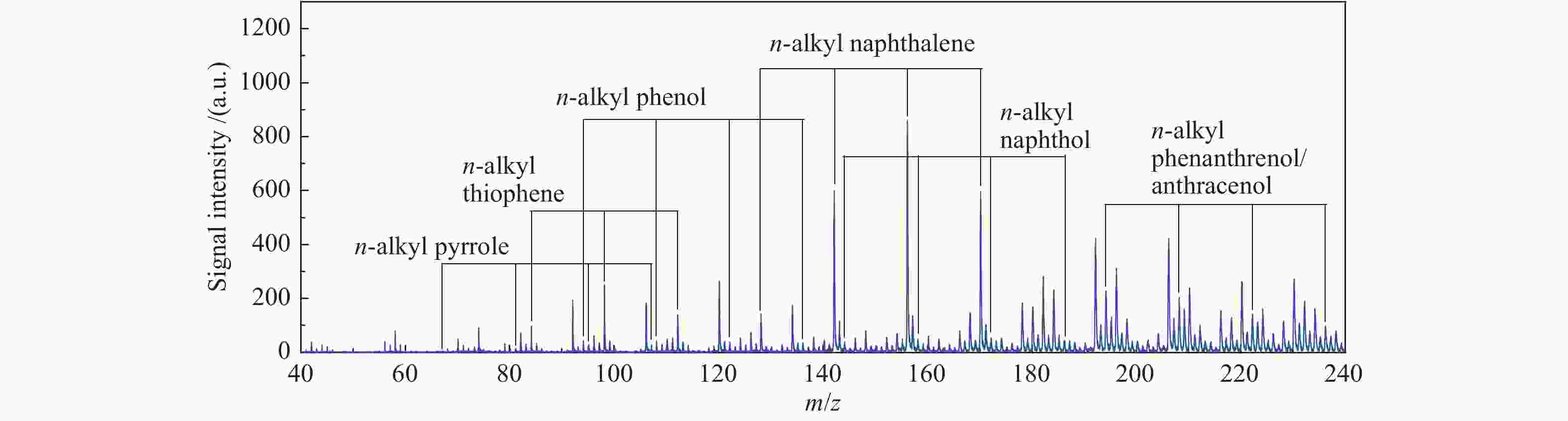
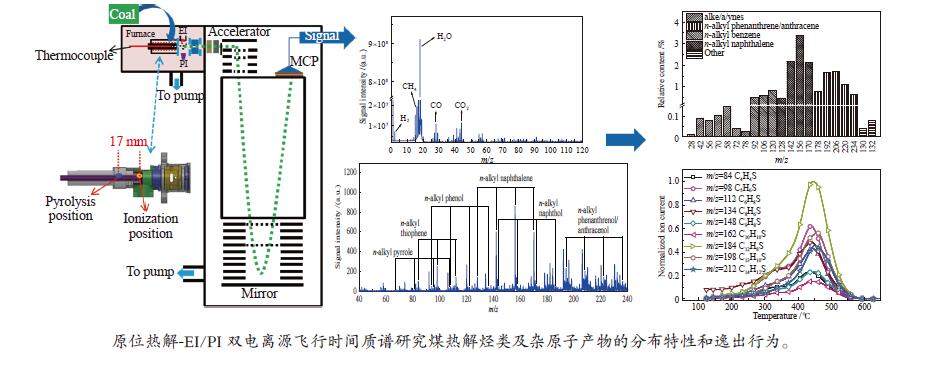
In-situ characterization of volatiles from pyrolysis of Fengfeng coal by a double ionization time-of-flight mass spectrometer
-
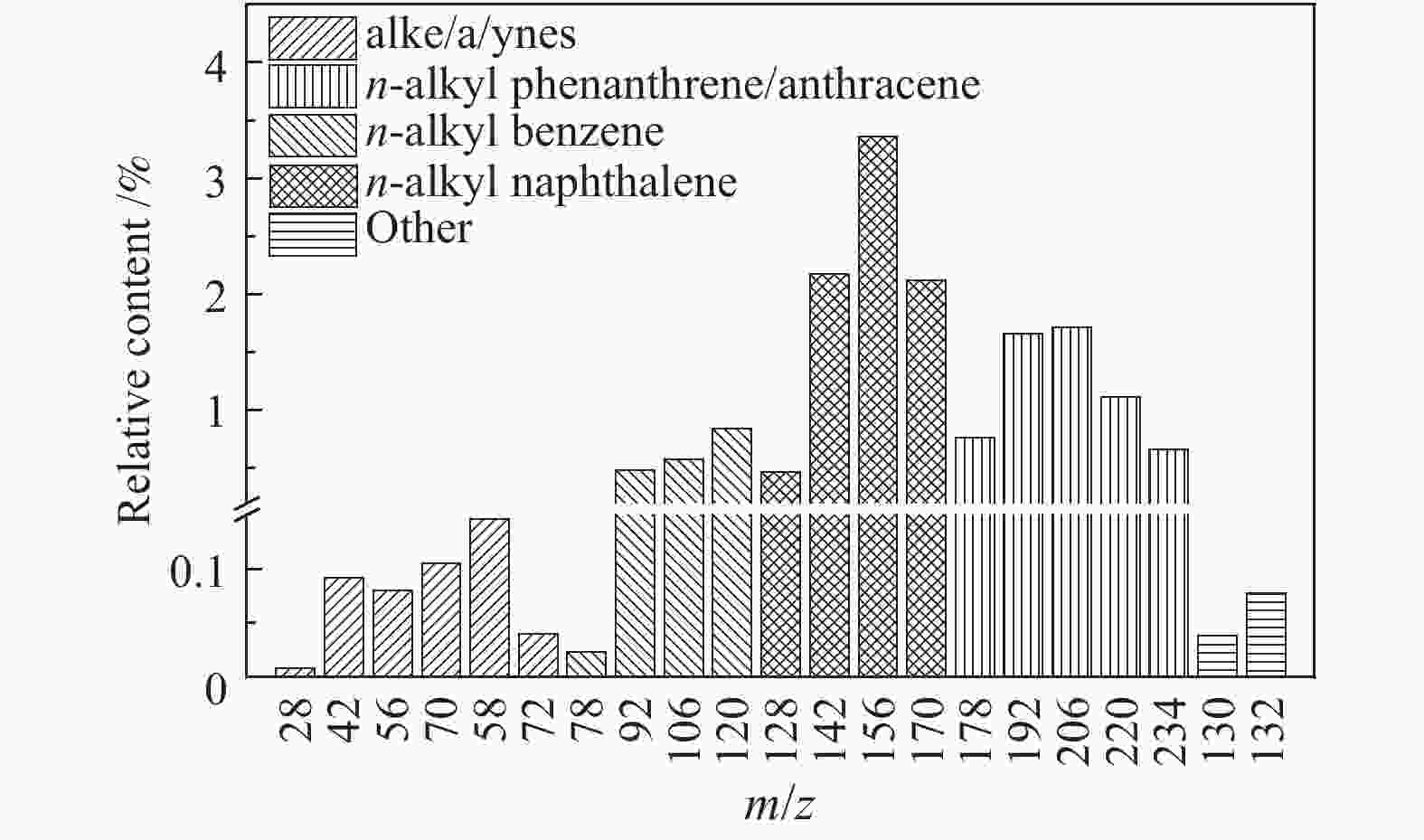
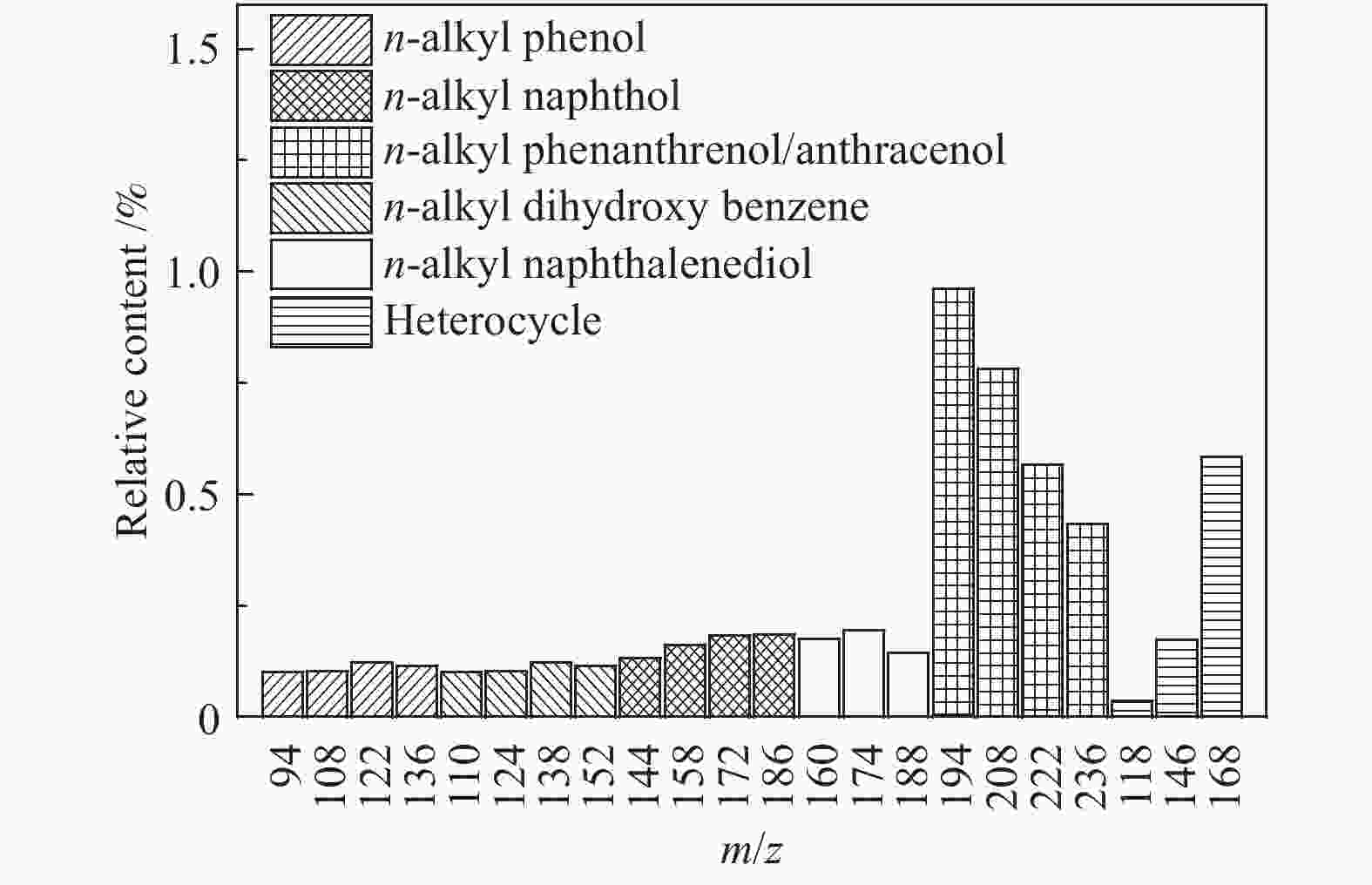
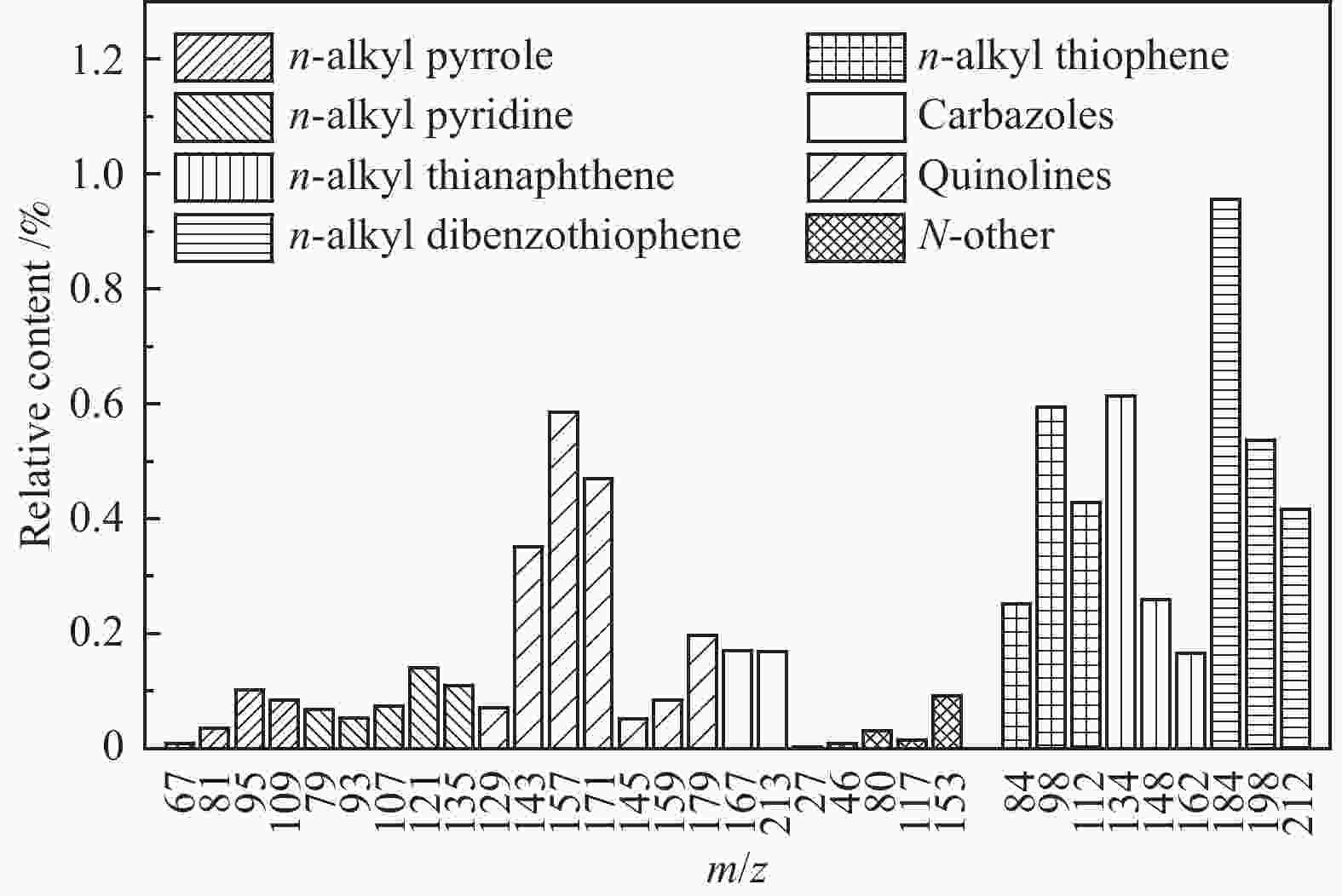
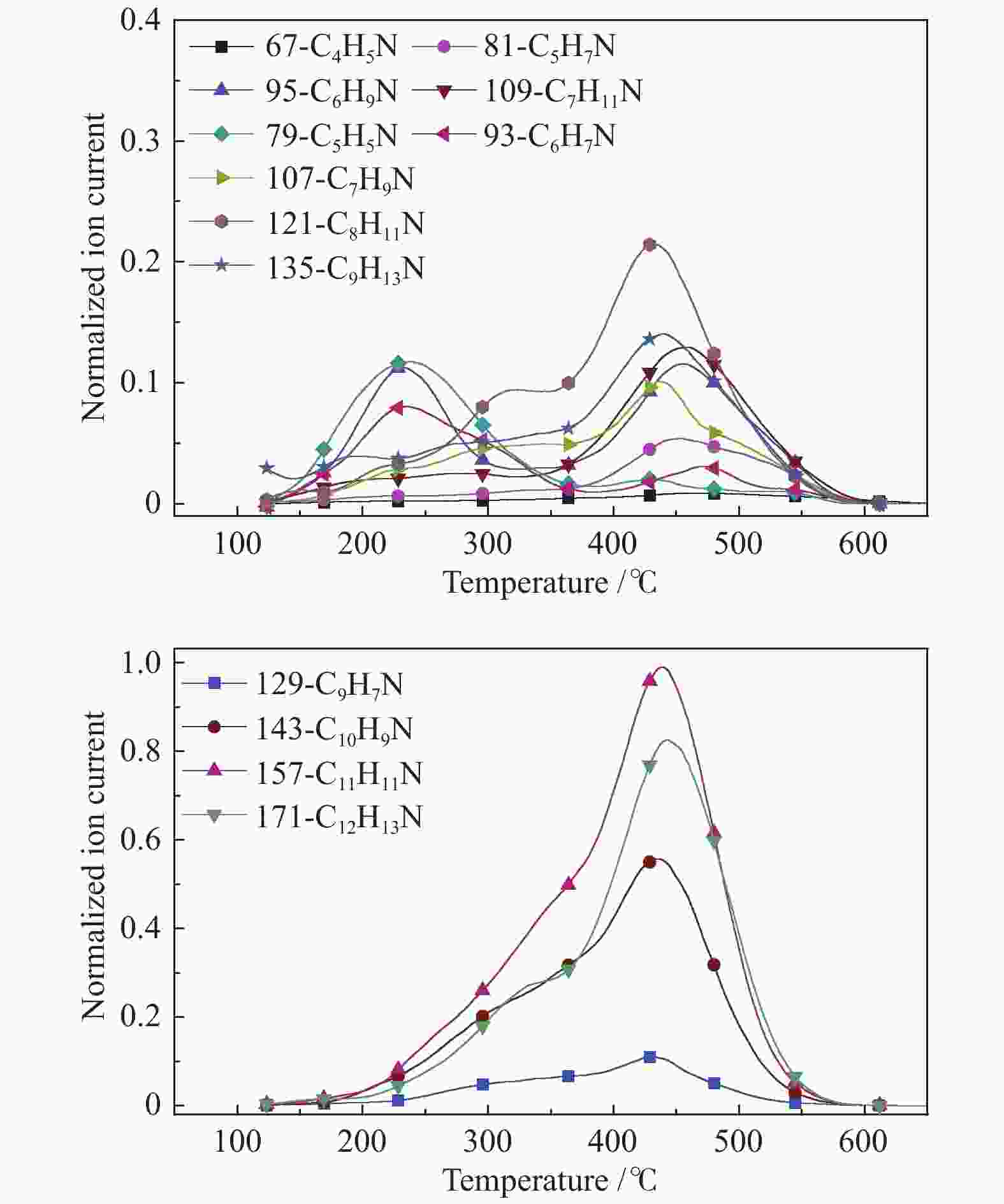
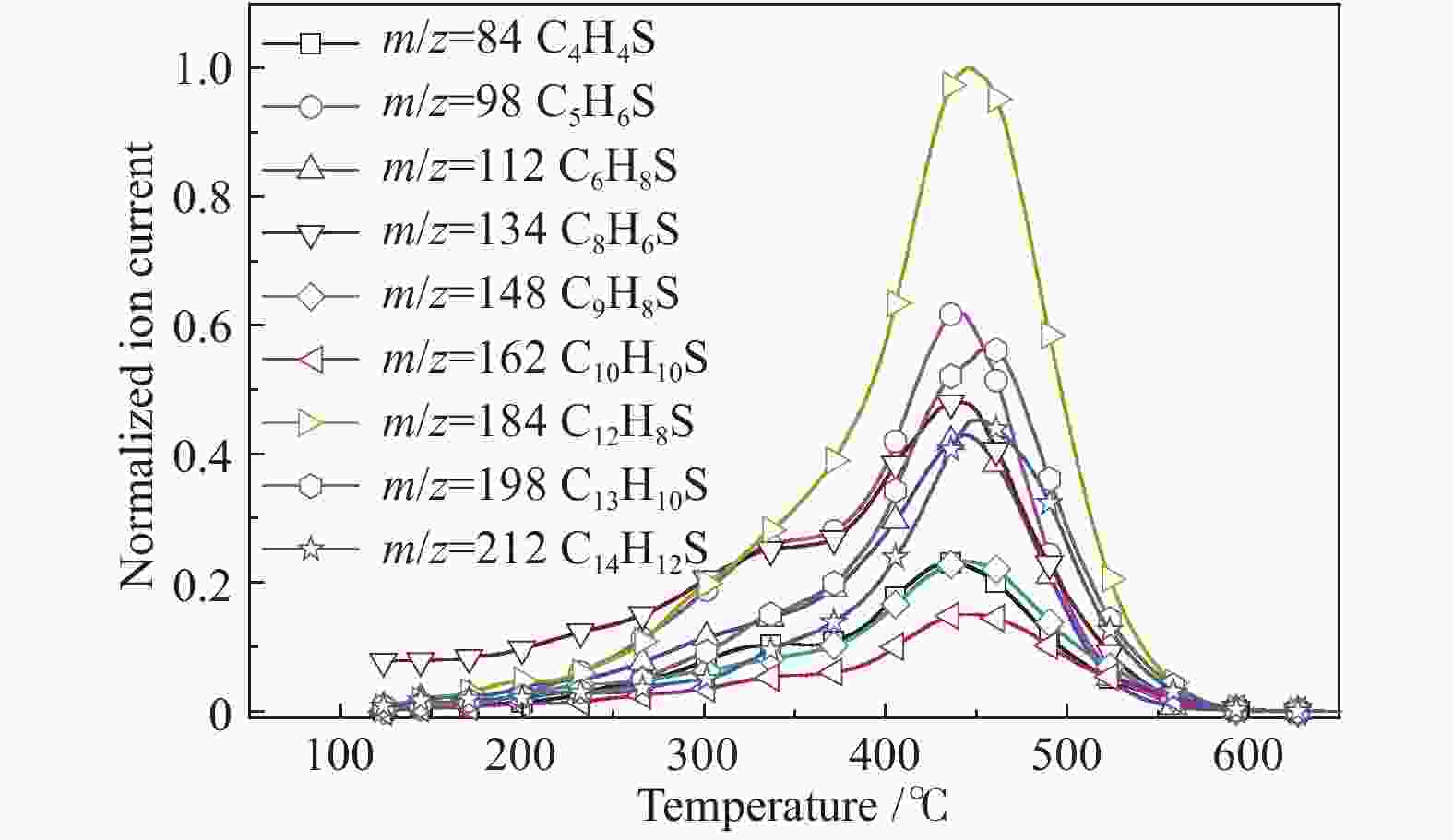
摘要: 利用自行搭建的原位热解-双电离源-飞行时间质谱(Py-EI/PI-TOFMS)对河北峰峰(FF)煤的热解行为,特别是含杂原子化合物的逸出特性进行研究。通过半定量分析获得产物的相对含量,通过对选定产物信号进行扫描获得其离子流强度随温度的变化曲线,同时利用电子轰击分析了H2O、CO、CO2、H2和CH4五种小分子气体产物的逸出规律。结果表明,该原位热解-飞行时间质谱系统很好地实现了煤热解过程中初级产物的原位检测与表征。对占检测到的挥发分约70%的质荷比小于240的热解产物的相对含量分布进行分析发现,烃类产物以1至3环芳烃为主;酚类化合物以含1至3个苯环的酚为主,其中,含3个苯环的酚类化合物含量明显高于含1至2个苯环的;含相同烷基取代基的酚类化合物的最大逸出温度随苯环数的增加向低温移动。热解挥发分中含氮/硫化合物的相对含量均小于1.0%,硫杂环化合物的含量高于吡咯/吡啶类化合物。Abstract: Coal from Fengfeng (FF) colliery, Hebei, China, was pyrolyzed in a self-developed pyrolysis reactor coupled with double ionization sources (viz., electron impact ionization (EI) and photoionization (PI)) and time-of-flight mass spectrometer (Py-EI/PI-TOFMS), to characterize in-situ the primary products from pyrolysis (heteroatom-containing compounds in particular). The relative contents of various pyrolysis products were obtained by semi-quantitative analysis and the temperature-evolved profile of each product was obtained by scanning the signal of the distinguished peak with the lapse of time; in addition, the evolution of five small molecule gaseous products (viz., H2O, CO, CO2, H2 and CH4) was analyzed by EI-TOFMS. The results indicate that the Py-PI-TOFMS system is able to detect and characterize the primary products in-situ during coal pyrolysis. In the pyrolysis products with a mass-to-charge ratio (m/z) less than 240, which account for about 70% of detected volatiles, hydrocarbons consist of mainly aromatics of 1–3 rings, whereas the phenolic compounds are dominated by phenols containing 1–3 benzene rings (especially the 3-ring phenols). The peak temperature with maximum evolution of phenols containing the same alkyl substituents shifts to lower temperature with the increase of the number of aromatic rings. In addition, the relative contents of nitrogen/sulfur-containing compounds are all less than 1.0%, where the content of sulfur-containing heterocyclic compounds is higher than that of nitrogen-containing heterocyclic compounds.
-
表 1 峰峰煤的工业分析和元素分析
Table 1 Proximate and ultimate analyses of FF coal
Proximate analysis w/% Ultimate analysis wdaf/% Mad Ad Vdaf FCdaf C H N S O* 0.05 10.6 31.54 68.46 83.59 3.08 1.69 0.69 10.95 表 2 FF煤初始热解产物的归属
Table 2 Mass assignment of primary pyrolysis products from FF coal
m/z Name Formula IE/eV m/z Name Formula IE/eV 28 ethylene C2H4 10.51 128 naphthalene C10H8 8.14 42 propylene C3H6 9.73 142 methylnaphthalene C11H10 7.96 56 butene C4H8 9.10 156 dimethylnaphthalene/ethyl naphthalene C12H12 8.11/7.95 70 pentene C5H10 9.04 170 C3 alkyl naphthalene C13H14 58 butane C4H10 10.55 130 1,2-dihydronaphthalene C10H10 8.14 72 C5 alkanes C5H12 132 tetrahydronaphthalene C10H12 8.46 78 benzene C6H6 9.24 178 phenanthrene/anthracene C14H10 7.44/7.899 92 toluene C7H8 8.83 192 methyl phenanthrene/anthracene C15H12 7.90/7.70 106 dimethyl benzene/ethyl benzene C8H10 8.44/8.77 206 C2 alkyl phenanthrene/anthracene C16H14 7.53−8.01 120 C3 alkyl benzene C9H12 220 C3 alkyl phenanthrene/anthracene C17H16 68 furan C4H4O 8.89 144 naphthol C10H8O 7.89 146 coumarin C9H6O2 8.72 158 methyl naphthol C11H10O 7.82 118 benzofuran C8H6O 8.80 172 dimethyl naphthol/ethyl naphthol C12H12O 168 dibenzofuran C12H8O 8.09 186 C3 alkyl naphthol C13H14O 94 phenol C6H6O 8.49 160 naphthalenediol C10H8O2 7.62 108 cresol C7H8O 8.29 174 methyl naphthalenediol C11H12O2 122 dimethyl phenol/ethyl phenol C8H10O 188 C2 alkyl naphthalenediol C12H14O2 136 C3 alkyl phenol C9H12O 202 C3 alkyl naphthalenediol C13H16O2 110 benzenediol C6H6O2 7.94 194 phenanthrenol/anthracenol C14H10O 8.83 124 methyl benzenediol C7H8O2 208 methyl phenanthrenol/anthracenol C15H12O 138 dimethyl benzenediol/ethyl benzenediol C8H10O2 222 C2 alkyl phenanthrenol/anthracenol C16H14O 152 C3 alkyl benzenediol C9H12O2 236 C3 alkyl phenanthrenol/anthracenol C17H16O 145 4-hydroxy-quinoline C9H7NO 8.20 159 2-hydroxy-7-methyl-quinoline C10H9NO 67 pyrrole C4H5N 8.21 80 pyrimidine C4H4N2 8.71 81 methyl pyrrole C5H7N 8.01 129 quinoline C9H7N 8.30 95 C2 alkyl pyrrole C6H9N 143 methyl quinoline C10H9N 109 C3 alkyl pyrrole C7H11N 157 dimethyl quinoline/ethyl quinoline C11H11N 79 pyridine C5H5N 9.25 171 C3 alkyl quinoline C12H13N 93 methyl pyridine C6H7N 9.02 179 benzoquinoline C13H9N 7.80 107 dimethyl pyridine/ethyl pyridine C7H9N 8.85/ 167 carbazole C12H9N 7.57 121 C3 alkyl pyridine C8H11N 213 benzocarbazole C16H11N 7.10 117 indole C8H7N 7.76 134 thianaphthene C8H6S 8.13 153 naphthalene nitrile C11H7N 8.64 148 methyl thianaphthene C9H8S 84 thiophene C4H4S 8.86 162 dimethyl thianaphthene C10H10S 98 methyl thiophene C5H6S 8.59 184 dibenzothiophene C12H8S 8.34 112 dimethyl thiophene C6H8S 8.23 198 methyl dibenzothiophene C13H10S 212 dimethyl dibenzothiophene C14H12S 8.77 表 3 酚类化合物的最大逸出峰温度
Table 3 Peak temperatures with maximum evolution of phenols during the pyrolysis of FF coal
Mono-
phenolPhenol Naphthol Phenanthrenol/
anthracenolC0 C1 C2 C3 C0 C1 C2 C3 C0 C1 C2 C3 tMax/℃ 466 501 475 469 452 461 466 467 450 458 461 459 Bis-
phenolsbenzenediol naphthalenediol C0 C1 C2 C0 C1 C2 tMax/℃ 458 462 457 450 448 456 表 4 不同芳醚ArO-CH3键的解离能 (kcal/mol)
Table 4 Bond dissociation energies (BDEs) of ArO–CH3 bonds in different aromatic ethers (kcal/mol)
Structure B3LYP Structure B3LYP Structure B3LYP Structure B3LYP 
54.59 
54.89 
49.26 
49.22 
53.92 
53.64 
47.11 
47.09 
50.14 
50.51 
43.90 
43.89 -
[1] STOCK L M. Coal pyrolysis[J]. Acc Chem Res,1989,22:427−433. doi: 10.1021/ar00168a004 [2] MORGAN T J, KANDIYOTI R. Pyrolysis of coals and biomass: Analysis of thermal breakdown and its products[J]. Chem Rev,2014,114:1547−1607. doi: 10.1021/cr400194p [3] KONG J, ZHAO R F, BAI Y H, LI G L, ZHANG C, LI F. Study on the formation of phenols during coal flash pyrolysis using pyrolysis-GC/MS[J]. Fuel Process Technol,2014,127:41−46. doi: 10.1016/j.fuproc.2014.06.004 [4] MAHAT R K, RODGERS W, BASILE F. Microwave radiation heating in pressurized vessels for the rapid extraction of coal samples for broad spectrum GC-MS analysis[J]. Energy Fuels,2014,28:6326−6335. doi: 10.1021/ef501659h [5] LIEVENS C, CI D H, BAI Y, MA L G, ZHANG R, CHEN J Y, GAI Q Q, LONG Y H, GUO X F. A study of slow pyrolysis of one low rank coal via pyrolysis–GC/MS[J]. Fuel Process Technol,2013,116:85−93. doi: 10.1016/j.fuproc.2013.04.026 [6] ARENILLAS A, RUBIERA F, PIS J J. Simultaneous thermogravimetric-mass spectrometric study on the pyrolysis behaviour of different rank coals[J]. J Anal Appl Pyrolysis,1999,50:31−46. doi: 10.1016/S0165-2370(99)00024-8 [7] MIURA K. Mild conversion of coal for producing valuable chemicals[J]. Fuel Process Technol,2000,62:119−135. doi: 10.1016/S0378-3820(99)00123-X [8] NOLA G D, DEJONG W, H S. TG-FTIR characterization of coal and biomass single fuels and blends under slow heating rate conditions: Partitioning of the fuel-bound nitrogen[J]. Fuel Process Technol,2010,91:103−115. doi: 10.1016/j.fuproc.2009.09.001 [9] POUTSMA M L. Free-radical thermolysis and hydrogenolysis of model hydrocarbons relevant to processing of coal[J]. Energy Fuels,1990,4:113−131. doi: 10.1021/ef00020a001 [10] MüHLBERGER F, STREIBEL T, WIESER J, ULRICH A, ZIMMERMANN R. Single photon ionization time-of-flight mass spectrometry with a pulsed electron beam pumped excimer VUV lamp for on-line gas analysis: Setup and first results on cigarette smoke and human breath[J]. Anal Chem,2005,77:7408−7414. doi: 10.1021/ac051194+ [11] MAMYRIN B A. Time-of-flight mass spectrometry[J]. Int J Mass Spectrom,2001,206:251−266. doi: 10.1016/S1387-3806(00)00392-4 [12] XU J Y, ZHUO J K, ZHU Y N, PAN Y, YAO Q. Analysis of volatile organic pyrolysis products of bituminous and anthracite coals with single-photon ionization time-of-flight mass spectrometry and gas chromatography/mass spectrometry[J]. Energy Fuels,2016,31:730−737. [13] SHI L, WANG X L, ZHANG S Y, WU X H, YUAN L, TANG Z C. A new in-situ pyrolytic time-of-flight mass spectrometer instrument for study on coal pyrolysis[J]. J Anal Appl Pyrolysis,2016,117:347−353. doi: 10.1016/j.jaap.2015.10.009 [14] LI G S, FAN X, YOU C Y, ZHAO Y P, WANG R Y, WEI X Y, MA F Y, LU X, MO W L, LI X. Molecular characteristics of the soluble components from three low-rank coals based on the analyses using GC/MS and GC/Q-TOF MS[J]. Fuel,2019,254:115602. [15] WANG F, FAN X, XIA J L, WEI X Y, YU Y R, ZHAO Y P, CAO J P, ZHAO W, WANG R Y. Insight into the structural features of low-rank coals using comprehensive two dimensional gas chromatography/time-of-flight mass spectrometry[J]. Fuel,2018,212:293−301. doi: 10.1016/j.fuel.2017.10.044 [16] CZECH H, SIPPULA O, KORTELAINEN M, TISSARI J, RADISCHAT C, PASSIG J, STREIBEL T, JOKINIEMI J, ZIMMERMANN R. On-line analysis of organic emissions from residential wood combustion with single-photon ionisation time-of-flight mass spectrometry (SPI-TOFMS)[J]. Fuel,2016,177:334−342. doi: 10.1016/j.fuel.2016.03.036 [17] ZHOU Z Y, LIU C J, CHEN X M, MA H, ZHOU C Q, WANG Y Z, QI F. On-line photoionization mass spectrometric study of lignin and lignite co-pyrolysis: Insight into the synergetic effect[J]. J Anal Appl Pyrolysis,2019,137:285−292. doi: 10.1016/j.jaap.2018.12.009 [18] LI G, LI L, JIN L J, TANG Z C, FAN H J, HU H Q. Experimental and theoretical investigation on three α,ω-diarylalkane pyrolysis[J]. Energy Fuels,2014,28:6905−6910. doi: 10.1021/ef502012b [19] ZHOU Y, LI L, JIN L.J, ZHOU J, SHI Z W, LI Y, HU H Q. Pyrolytic behavior of coal-related model compounds connected with C–C bridged linkages by in-situ pyrolysis vacuum ultraviolet photoionization mass spectrometry[J]. Fuel,2019,241:533−541. doi: 10.1016/j.fuel.2018.12.046 [20] JAKAB E, TILL F, VARHEGYI G. Thermogravimetric-mass spectrometric study on the low temperature oxidation of coals[J]. Fuel Process Technol,1991,28:221−238. [21] CAMPBELL J H. Pyrolysis of subbituminous coal in relation to in-situ gasification[J]. Fuel,1978,57:217−224. doi: 10.1016/0016-2361(78)90119-9 [22] HEEK K H V, HODEK W. Structure and pyrolysis behaviour of different coals and relevant model substances[J]. Fuel,1994,73:886−896. doi: 10.1016/0016-2361(94)90283-6 [23] CHARPENAY S, SERIO M A, BASSILAKIS R, SOLOMON P R. Influence of maturation on the pyrolysis products from coals and kerogens. 1. Experiment[J]. Energy Fuels,1996,10:19−25. doi: 10.1021/ef950149+ [24] SOLOMON P R, HAMBLEN D G, SERIO M A, YU Z Z, CHARPENAY S. A characterization method and model for predicting coal conversion behaviour[J]. Fuel,1993,72:469−488. doi: 10.1016/0016-2361(93)90106-C [25] IBARRA J V, MOLINER R, GAVILȦN M P. Functional group dependence of cross-linking reactions during pyrolysis of coal[J]. Fuel,1991,70:408−413. doi: 10.1016/0016-2361(91)90131-S [26] CHENG J, ZHANG Y S, WANG T, NORRIS P, CHEN W Y, PAN W P. Thermogravimetric-Fourier transform infrared spectroscopy-gas chromatography/mass spectrometry study of volatile organic compounds from coal pyrolysis[J]. Energy Fuels,2017,31:7042−7051. doi: 10.1021/acs.energyfuels.7b01073 [27] HE Q Q, WAN K J, HOADLEY A, YEASMIN H, MIAO Z Y. TG-GC-MS study of volatile products from Shengli lignite pyrolysis[J]. Fuel,2015,156:121−128. doi: 10.1016/j.fuel.2015.04.043 [28] WANG P F, JIN L J, LIU J H, ZHU S W, HU H Q. Analysis of coal tar derived from pyrolysis at different atmospheres[J]. Fuel,2013,104:14−21. doi: 10.1016/j.fuel.2010.06.041 [29] LINSTROM P J. NIST Chemistry Webbook [M]. http://webbooknistgov/chemistry/.2018. [30] LI G, ZHANG S Y, JIN L J, TANG Z C, HU H Q. In-situ analysis of volatile products from lignite pyrolysis with pyrolysis-vacuum ultraviolet photoionization and electron impact mass spectrometry[J]. Fuel Process Technol,2015,133:232−236. doi: 10.1016/j.fuproc.2015.02.016 [31] ZHOU Y, LI L, JIN L J, ZHU J L, LI J G, LI Y, FAN H J, HU H Q. Effect of functional groups on volatile evolution in coal pyrolysis process with in-situ pyrolysis photoionization time-of-flight mass spectrometry[J]. Fuel,2020,260:116322. [32] SUN Q L, LI W, CHEN H K, LI B Q. The variation of structural characteristics of macerals during pyrolysis[J]. Fuel,2003,82:669−676. doi: 10.1016/S0016-2361(02)00356-3 [33] 孙庆雷, 李文, 李东涛, 陈皓侃, 李保庆, 白向飞, 李文华. 神木煤有机显微组分的结构特征与热转化性质的关系[J]. 燃料化学学报,2003,31(2):97−102. doi: 10.3969/j.issn.0253-2409.2003.02.001SUN Qing-lei, LI Wen, LI Dong-tao, CHEN Hao-kan, LI Bao-qing, BAI Xiang-fei, LI Wen-hua. Relationship between structure characteristics and thermal conversion property of Shenmu maceral concentrates[J]. J Fuel Chem Technol,2003,31(2):97−102. doi: 10.3969/j.issn.0253-2409.2003.02.001 [34] DONG J, LI F, XIE K C. Study on the source of polycyclic aromatic hydrocarbons (PAHs) during coal pyrolysis by PY-GC-MS[J]. J Hazard Mater,2012,243:80−85. doi: 10.1016/j.jhazmat.2012.09.073 [35] MEYERS R A. Coal Structure[M]. London: Academic Press Inc., 1982. [36] BLAZSÓ M, JAKAB E. Study of thermal decomposition reactions in coals by pyrolysis-gas chromatography-mass spectrometry[J]. J Anal Appl Pyrolysis,1985,8:189−194. [37] YAN L J, BAI Y.H, ZHAO R F, LI F, XIE K C. Correlation between coal structure and release of the two organic compounds during pyrolysis[J]. Fuel,2015,145:12−17. doi: 10.1016/j.fuel.2014.12.056 [38] BUCKLEY A N. Nitrogen functionality in coals and coal-tar pitch determined by X-ray photoelectron spectroscopy[J]. Fuel Process Technol,1994,38:165−179. doi: 10.1016/0378-3820(94)90046-9 [39] BARTLE K D, PERRY D L, WALLACE S. The functionality of nitrogen in coal and derived liquids: An XPS study[J]. Fuel Process Technol,1987,15:350−361. [40] SOLOMON P R, COLKET M B. Evolution of fuel nitrogen in coal devolatilization[J]. Fuel,1978,57:749−755. doi: 10.1016/0016-2361(78)90133-3 [41] KELEMEN S R, GORBATY M L, KWIATEK P J, FLETCHER T H, WATT M, SOLUM M S, PUGMIRE R J. Nitrogen transformations in coal during pyrolysis[J]. Energy Fuels,1998,12:159−173. doi: 10.1021/ef9701246 [42] 陈鹏. 用XPS研究兖州煤各显微组分中有机硫存在形态[J]. 燃料化学学报,1997,25(3):238−241.CHEN Peng. Application of XPS in study dormation of organic sulfur in macerals of Yanzhou coal[J]. J Fuel Chem Technol,1997,25(3):238−241. [43] 李梅, 杨俊和, 张启锋, 常海洲, 孙慧. 用 XPS研究新西兰高硫煤热解过程中氮、硫官能团的转变规律[J], 燃料化学学报, 2013, 41(11): 1287−1293.LI Mei, YANG Jun-he, ZHANG Qi-feng, CHANG Hai-zhou, SUN Hui. XPS study on transformation of N- and S- functional groups during pyrolysis of high sulfur New Zealand coal[J]. J Fuel Chem Technol, 2013, 41(11): 1287−1293. [44] ZHAO Y P, HU H Q, JIN L J, HE X F, WU B. Pyrolysis behavior of vitrinite and inertinite from Chinese Pingshuo coal by TG–MS and in a fixed bed reactor[J]. Fuel Process Technol,2011,92:780−786. doi: 10.1016/j.fuproc.2010.09.005 [45] XING M W, KONG J, DONG J, JIAO H L, LI F. Thiophenic sulfur compounds released during coal pyrolysis[J]. Environ Eng Sci,2013,30:273−279. doi: 10.1089/ees.2011.0540 -




 下载:
下载: