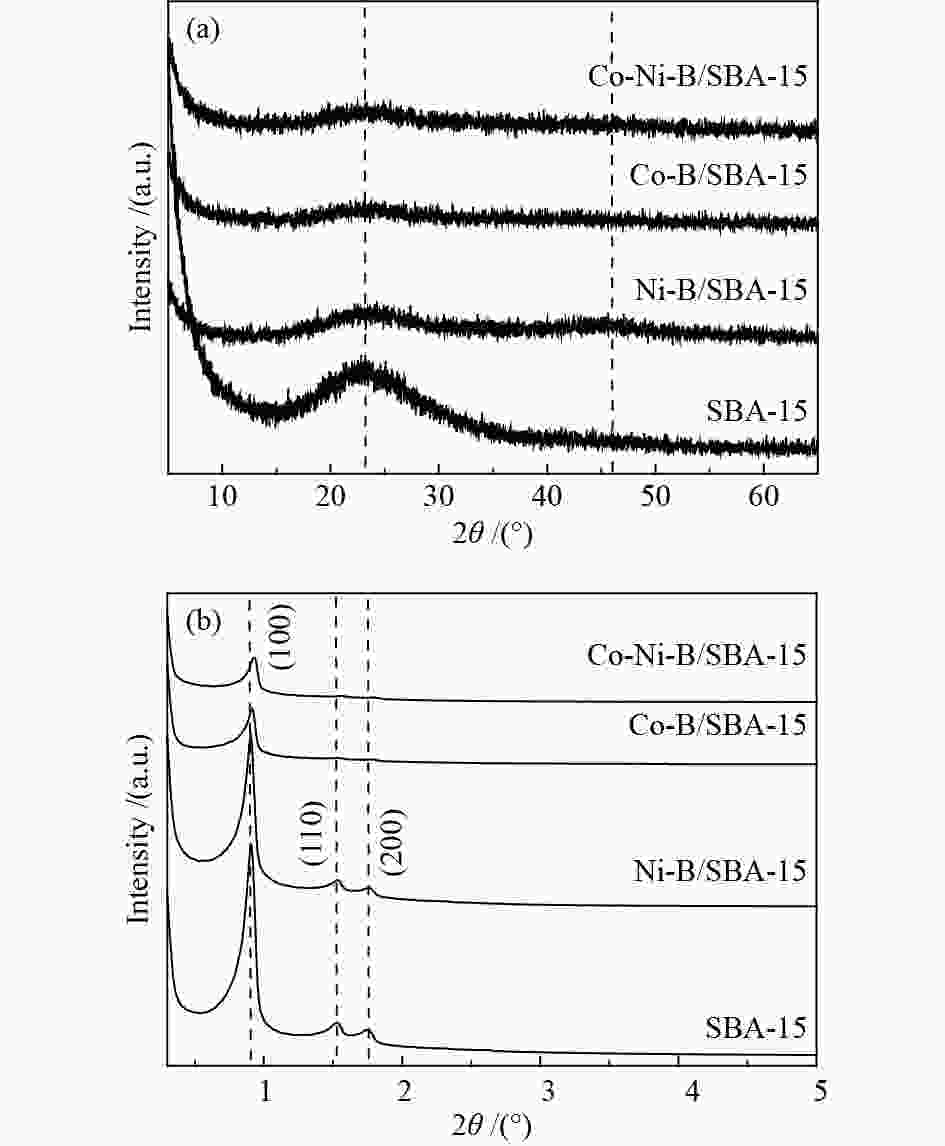
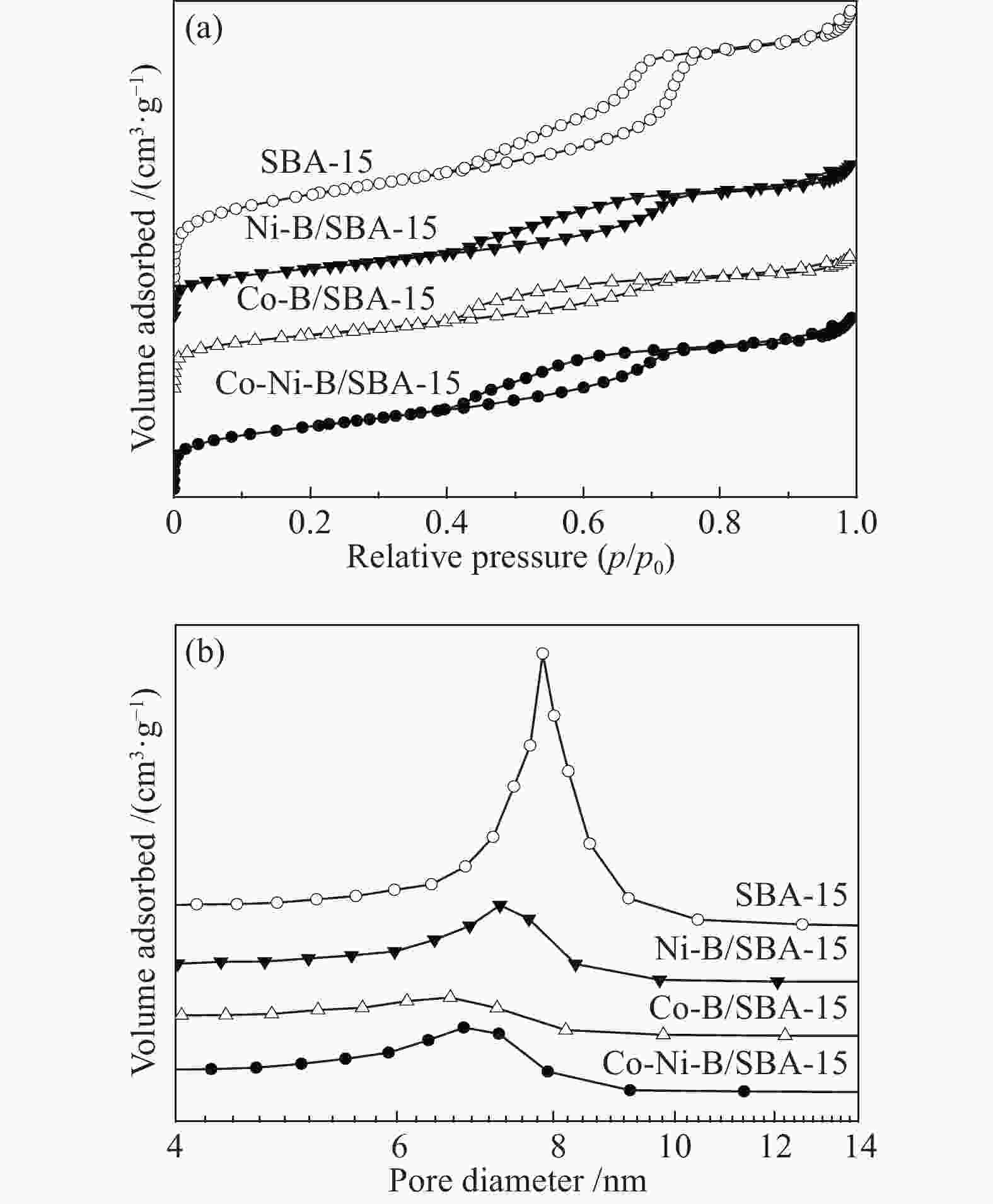
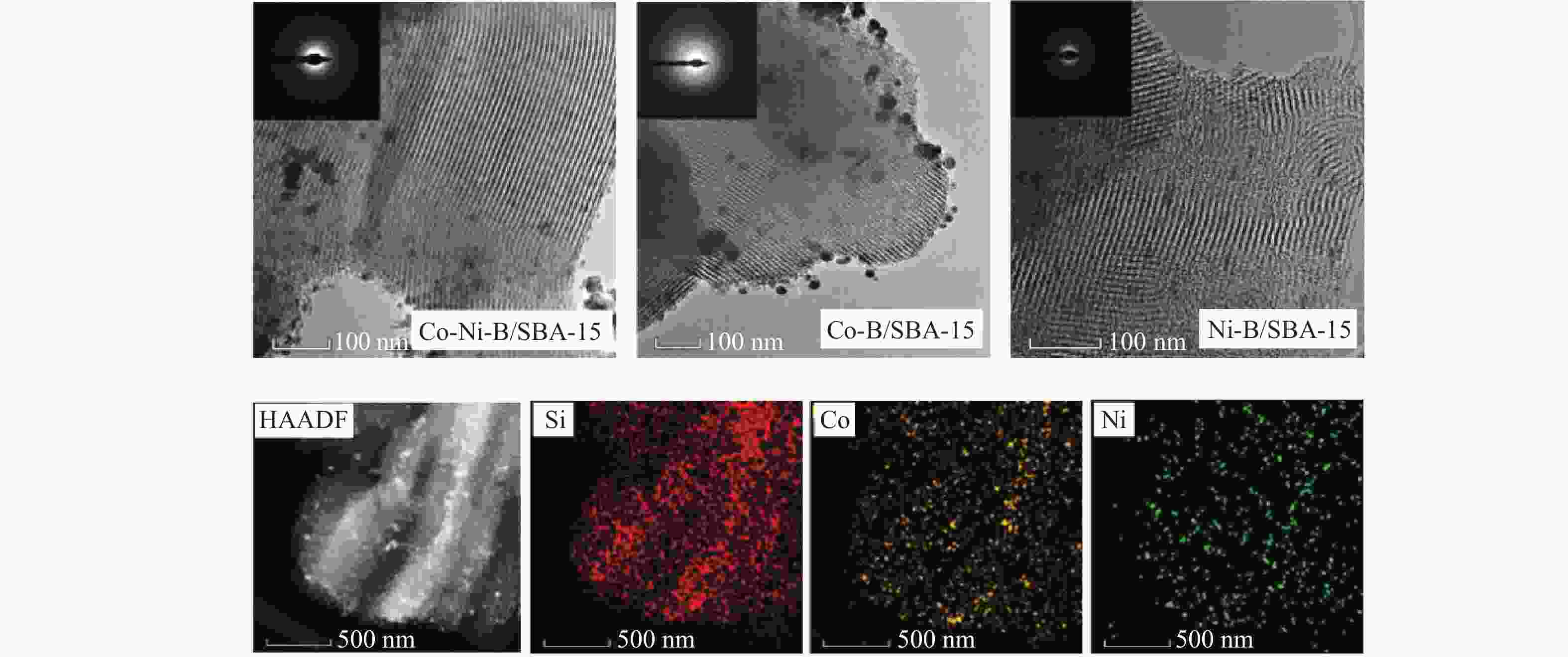
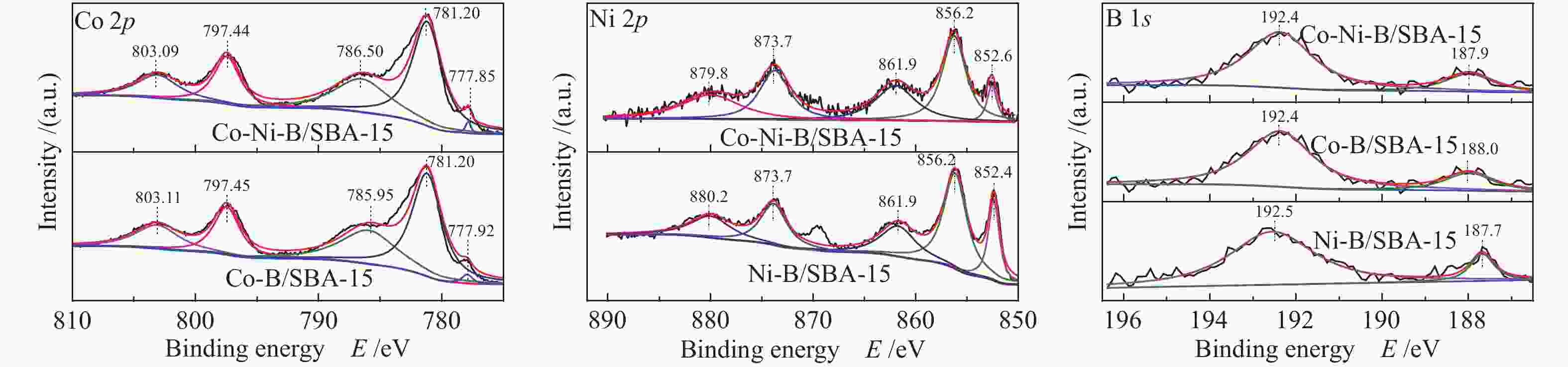
-
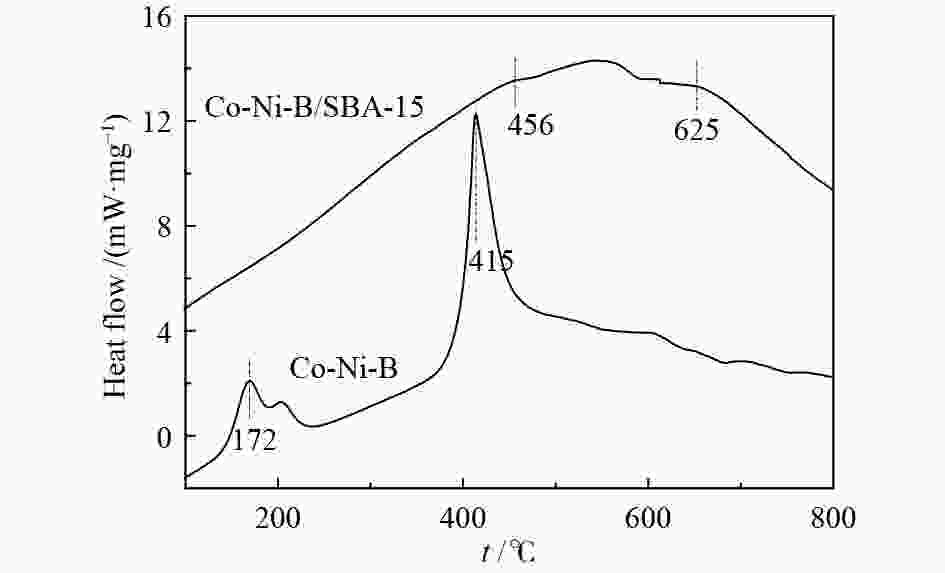
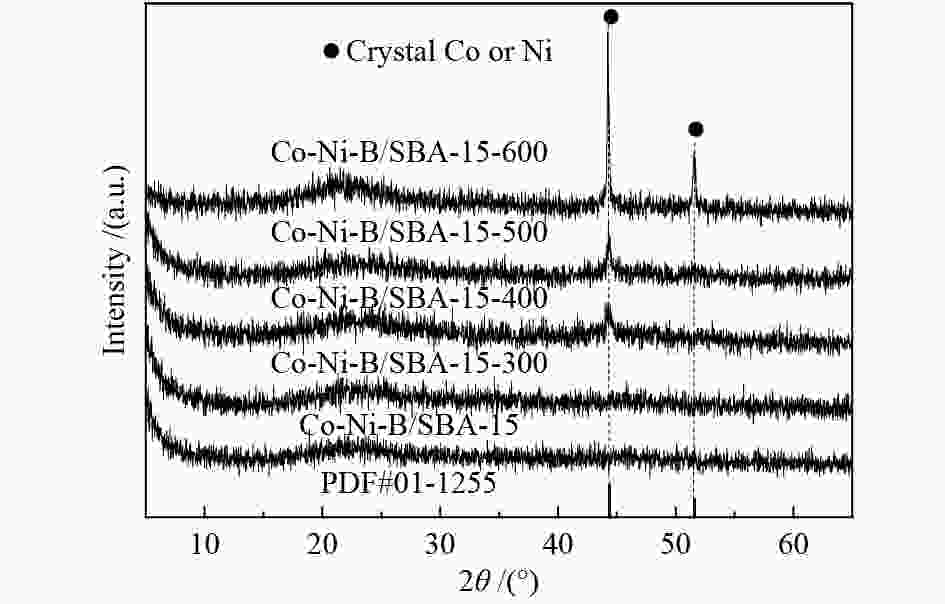
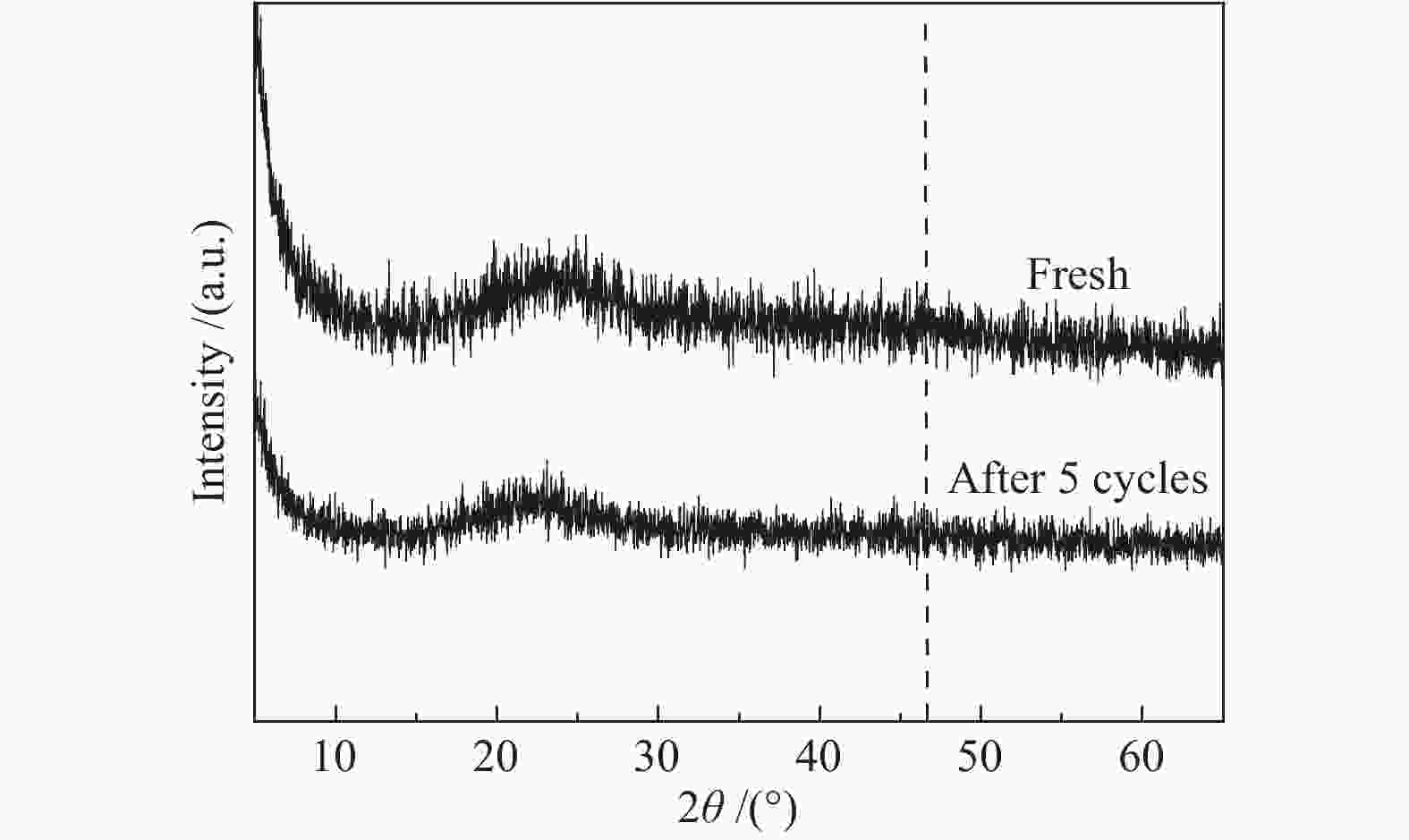
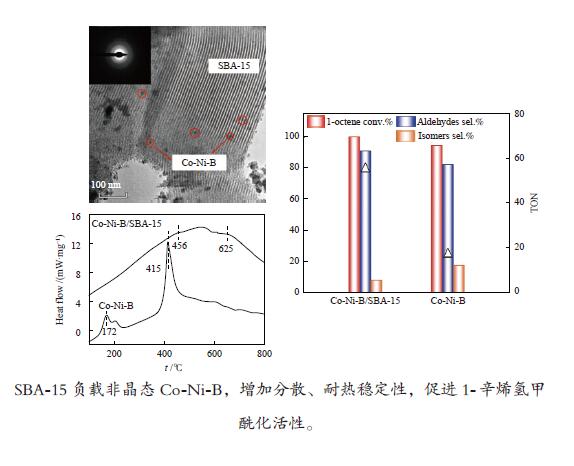
摘要: 超声辅助浸渍法制备了SBA-15分子筛负载三组分非晶态Co-Ni-B,研究了其对1-辛烯氢甲酰化制壬醛反应的催化性能。SBA-15负载纳米非晶态Co-Ni-B,增加了Co-Ni-B的分散;且非晶态Co-Ni-B转化为晶体的温度升高约280 ℃,耐热稳定性增强。Co-Ni-B/SBA-15催化剂,其中,Co = 17.22%,n(Ni)/n(Co) = 0.157,n(B)/n(Co + Ni) = 0.434,用于120 ℃、5 MPa条件下氢甲酰化反应,间歇反应4 h,1-辛烯完全转化,壬醛选择性91.24%;相比Co-Ni-B,副产物减少一倍。催化剂重复使用五次,活性稳定。Abstract: SBA-15 zeolite supported amorphous ternary Co-Ni-B was prepared by the ultrasonic-assisted impregnation method for catalyzing the hydroformylation of 1-octene to nonanal. Supporting on SBA-15 increased the dispersion of nano amorphous Co-Ni-B. The crystallization temperature of amorphous Co-Ni-B was elevated by 280 ℃ that improves the thermal stability of Co-Ni-B. Using Co-Ni-B/SBA-15 with Co loading of 17.22% and the ratio of n(Ni)/n(Co) of 0.157 and n(B)/n(Co + Ni) of 0.434, the conversion of 1-octene and the selectivity of nonanal reached 100% and 91.24%, respectively, in the hydroformylation reaction at 120 °C and 5 MPa. The selectivity of side-product was reduced by 1 time compared with that of Co-Ni-B. The Co-Ni-B/SBA-15 is stable after repeated used for 5 times.
-
Key words:
- Co-Ni-B /
- SBA-15 /
- 1-octene /
- hydroformylation /
- catalytic activity
-
表 1 催化剂的织构性质
Table 1 Textural properties of catalysts
Catalyst Real bulk w/% Real loading w/% ABET/(m2·g−1) vpore/(cm3·g−1) Dpore/nm Co Ni B SBA-15 − − − − 713 1.03 7.8 Co-Ni-B 63.52 10.74 7.25 − 22.9 0.15 26.6 Co-Ni-B/SBA-15 17.22 2.69 1.59 21.50 539 0.64 6.8 Co-B/SBA-15 15.99 − 1.28 17.27 453 0.49 6.6 Ni-B/SBA-15 − 19.03 1.65 20.68 413 0.57 7.3 表 2 1-辛烯氢甲酰化催化活性
Table 2 Catalytic activity for hydroformylation of 1-octene
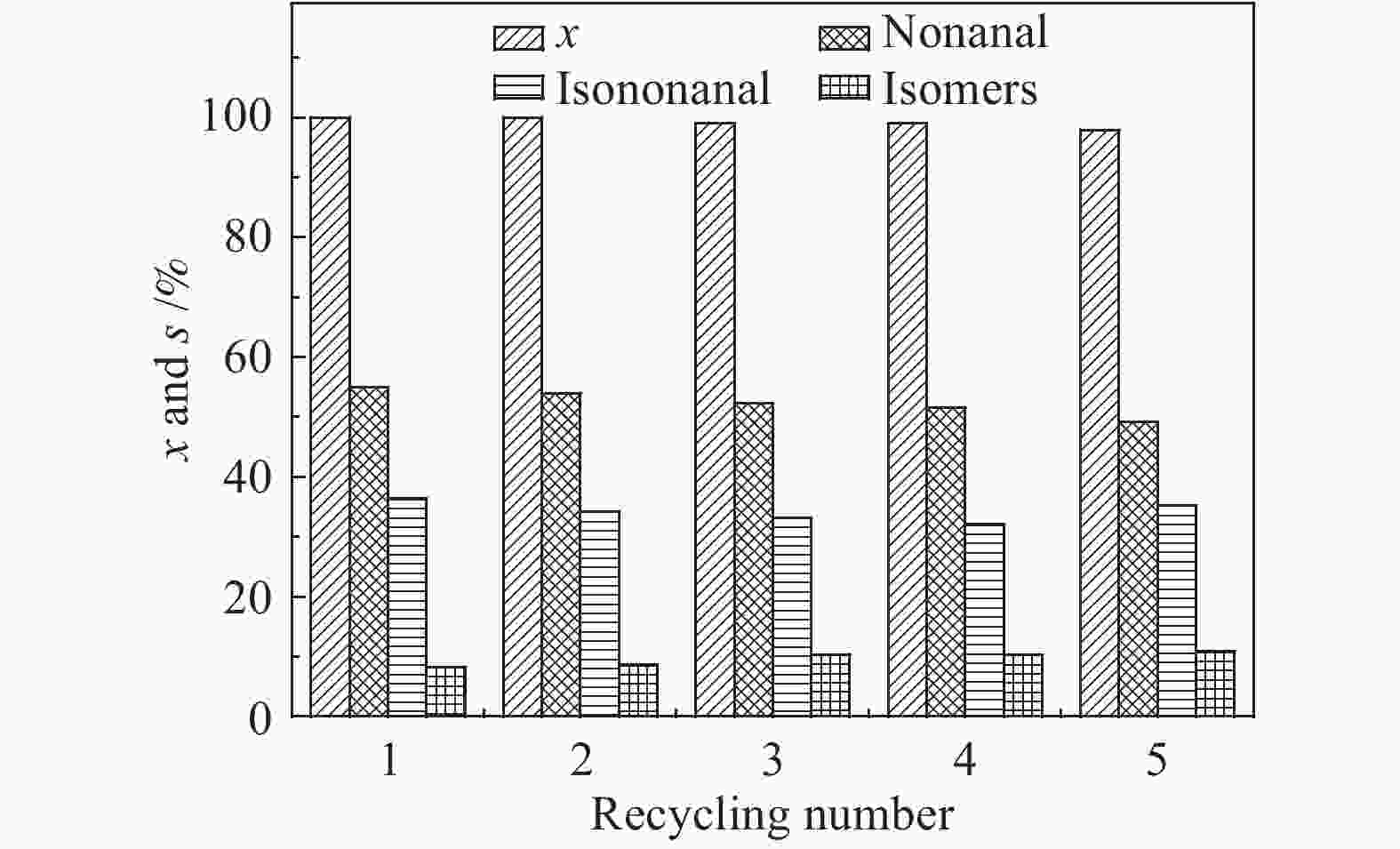
Catalyst Co/1-octene(molar ratio) x/% Product s/% Aldehyde yield/% n/i TON nonanal isononanal isomers others Co-Ni-B/SBA-15 0.018 100 54.89 36.35 8.29 0.47 91.24 1.51 55.5 Co-B/SBA-15 0.019 100 50.64 34.30 10.02 5.04 84.94 1.48 52.3 Ni-B/SBA-15 − 5.81 27.63 11.51 46.28 14.58 2.27 2.40 3.3 Co-Ni-B 0.055 94.63 49.48 32.99 17.53 0 78.04 1.59 17.3 cat. 0.25 g, toluene 13 g, 1-octene 3.57 g, 120 ℃, 5 MPa, 1200 r/min, 4 h 表 3 焙烧对催化活性的影响
Table 3 Effect of calcination on catalytic activity
Calc. t/℃ x/% Product s/% Aldehyde yield/% n/i TON nonanal isononanal isomers others Uncalcined 100 54.89 36.35 8.29 0.47 91.24 1.51 55.5 300 100 47.49 31.85 12.59 8.07 79.34 1.49 55.1 400 97.15 49.86 30.49 17.08 2.57 78.06 1.59 53.2 500 92.67 36.54 24.03 16.97 22.46 56.13 1.52 50.7 600 24.62 35.03 21.23 18.61 25.13 13.85 1.65 13.5 Co-Ni-B/SBA-15 0.25 g, toluene 13 g, 1-octene 3.57 g, 120 ℃, 5 MPa, 1200 r/min, 4 h -
[1] 姜淼, 杜虹, 王国庆, 严丽, 丁云杰. Co-PPh3@POPs多相催化剂氢甲酰化反应研究[J]. 煤炭学报,2020,45(4):1250−1258.JIANG Miao, DU Hong, WANG Guo-qing, YAN Li, DING Yun-jie. Co-PPh3@POPs heterogeneous catalysts for hydroformylation of olefins[J]. J China Coal Soc,2020,45(4):1250−1258. [2] SUDHEESH N, SHARMA S K, SHUKLA R S, JASRA R V. HRh(CO)(PPh3)3 encapsulated mesopores of hexagonal mesoporous silica (HMS) acting as nanophase reactors for effective catalytic hydroformylation of olefins[J]. J Mol Catal A: Chem,2008,296(1/2):61−70. doi: 10.1016/j.molcata.2008.08.019 [3] LIU Y, LI Z H, WANG B, ZHANG Y. A fine dispersed cobalt catalyst with macro-pore for hydroformylation of 1-hexene[J]. Catal Lett,2016,146(11):2252−2260. doi: 10.1007/s10562-016-1853-z [4] TAN M H, YANG G H, WANG T J, VITIDSANT T, LI J, WEI Q H, AI P P, WU M B, ZHENG J T, TSUBAKI N. Active and regioselective rhodium catalyst supported on reduced graphene oxide for 1-hexene hydroformylation[J]. Catal Sci Technol,2016,6(4):1162−1172. doi: 10.1039/C5CY01355K [5] LI C Y, SUN K J, WANG W L, YAN L, SUN X P, WANG Y Q, XIONG K, ZHAN Z P, JIANG Z, DING Y J. Xantphos doped Rh/POPs-PPh3 catalyst for highly selective long-chain olefins hydroformylation: Chemical and DFT insights into Rh location and the roles of Xantphos and PPh3[J]. J Catal,2017,353:123−132. doi: 10.1016/j.jcat.2017.07.022 [6] 吴丹, 周聪, 赵素英. 负载型烯烃氢甲酰化反应催化剂研究进展[J]. 化工进展,2019,38(10):4542−4553.WU Dan, ZHOU Cong, ZHAO Su-Ying. Research progress of immobilized catalysts for olefin hydroformylation[J]. Chem Ind Eng Prog,2019,38(10):4542−4553. [7] SONG X, DING Y, CHEN W, DONG W, PEI Y, ZANG J, YAN L, LU Y. Formation of 3-pentanone via ethylene hydroformylation over Co/activated carbon catalyst[J]. Appl Catal A: Gen,2013,452:155−162. doi: 10.1016/j.apcata.2012.11.006 [8] QIU X, TSUBAKI N, FUJIMOTO K. Hydroformylation of 1-hexene over Co/SiO2 catalysts: influence of pore size of support[J]. J Chem Eng Jpn,2001,34(11):1366−1372. doi: 10.1252/jcej.34.1366 [9] ZHANG J, SUN P, GAO G, WANG J, ZHAO Z L, MUHAMMAD Y, LI F W. Enhancing regioselectivity via tuning the microenvironment in heterogeneous hydroformylation of olefins[J]. J Catal,2020,387:196−206. doi: 10.1016/j.jcat.2020.03.032 [10] EPHRAIM V, PHENDUKANI N, KALALA J, REINOUT M. Confinement effect of rhodium (I) complex species on mesoporous MCM-41 and SBA-15: effect of pore size on the hydroformylation of 1-octene[J]. J Porous Mater,2017,25(1):303−320. [11] MARRAS F, WANG J, COPPENS M O, REEK J N H. Ordered mesoporous materials as solid supports for rhodium-diphosphine catalysts with remarkable hydroformylation activity[J]. Chem Commun,2010,46(35):6587−6589. doi: 10.1039/c0cc00924e [12] LI C Y, XIONG K, YAN L, JIANG M, SONG X G, WANG T, CHEN X K, ZHAN Z P, DING Y J. Designing highly efficient Rh/CPOL-bp& PPh3 heterogenous catalysts for hydroformylation of internal and terminal olefins[J]. Catal Sci Technol,2016,6(7):2143−2149. doi: 10.1039/C5CY01655J [13] LANG R, LI T, MATSUMURA D, MIAO S, REN Y J, CUI Y T, TAN Y, QIAO B T, LI L, WANG A Q, WANG X D, ZHANG T. Hydroformylation of olefins by a rhodium single-atom catalyst with activity comparable to RhCl(PPh3)3[J]. Angew Chem Int Ed,2016,55(52):16054−16058. doi: 10.1002/anie.201607885 [14] WANG L B, ZHANG W B, WANG S P, GAO Z H, LUO Z H, WANG X, ZENG R, LI A W, LI H L, WANG M L, ZHENG X S, ZHU J F, ZHANG W H, MA C, SI R, ZENG J. Atomic-level insights in optimizing reaction paths for hydroformylation reaction over Rh/CoO single-atom catalyst[J]. Nat Commun,2016,7:14036. doi: 10.1038/ncomms14036 [15] MA L, PENG Q R, HE D H. Catalytic behaviors of amorphous Co-B catalysts in hydroformylation of 1-octene[J]. Catal Lett,2009,130(1/2):137−146. doi: 10.1007/s10562-009-9839-8 [16] 李金金, 马兰, 贺德华, 李光兴. 非晶态Co-P-B催化剂在1-辛烯氢甲酰化反应中的应用[J]. 高等学校化学学报,2011,32(12):2844−2848.LI Jin-jin, MA Lan, HE De-hua, LI Guang-xing. Hydroformylation of 1-octene over amorphous Co-P-B catalysts[J]. Chem J Chin Univ,2011,32(12):2844−2848. [17] SHI Y K, HU X J, ZHU B L, ZHANG S M, HUANG W P. Hydroformylation of 1-octene over nanotubular TiO2-supported amorphous Co-B catalysts[J]. Chem Res Chin Univ,2015,31(5):851−857. doi: 10.1007/s40242-015-5002-9 [18] LUO H S, LI H X, ZHUANG L. Furfural hydrogenation to furfuryl alcohol over a novel Ni-Co-B amorphous alloy catalyst[J]. Chem Lett,2001,30(5):404−405. doi: 10.1246/cl.2001.404 [19] 程庆彦, 刘栋杰, 王明明, 王延吉. Ni-Co-P非晶态合金催化香草醛HDO性能的研究[J]. 燃料化学学报,2019,47(10):1205−1213. doi: 10.3969/j.issn.0253-2409.2019.10.007CHENG Qing-yan, LIU Dong-jie, WANG Ming-ming, WANG Yan-ji. Study on catalytic performance of Ni-Co-P amorphous alloy for HDO of vanillin[J]. J Fuel Chem Technol,2019,47(10):1205−1213. doi: 10.3969/j.issn.0253-2409.2019.10.007 [20] WANG C, LIM S Y, DU G, LOEBICKI C Z, LI N, DERROUICHE S, HALLER G L. Synthesis, characterization, and catalytic performance of highly dispersed Co-SBA-15[J]. J Phys Chem C,2009,113(33):14863−14871. doi: 10.1021/jp901823v [21] WEI W, ZHAO Y, PENG S C, ZHANG H Y, BIAN Y P, LI H X, LI H. Hollow Ni-Co-B amorphous alloy nanospheres: Facile fabrication via vesicle-assisted chemical reduction and their enhanced catalytic performances[J]. J Mater Chem A,2014,2(45):19253−19259. doi: 10.1039/C4TA04533E [22] ZHAO J J, MALGRAS V, NA J, LIANG R, CAI Y, KANG Y Q, ALSHEHRI A A, ALZAHRANI K A, ALGHAMDI Y G, ASAHI T, ZHANG D Q, JIANG B, LI H X, YAMAUCHI Y. Magnetically induced synthesis of mesoporous amorphous CoB nanochains for efficient selective hydrogenation of cinnamaldehyde to cinnamyl alcohol[J]. Chem Eng J,2020,398:125564. [23] KANG Y Q, HENZIE J, GU H J, NA J, FATEHMULLA A, SHAMSAN B S A, ALDHAFIRI A M, FAROOQ W A, BANDO Y, ASAHI T, JIANG B, LI H X, YAMAUCHI Y. Mesoporous metal-metalloid amorphous alloys: the first synthesis of open 3D mesoporous Ni-B amorphous alloy spheres via a dual chemical reduction method[J]. Small,2020,16(10):1906707. doi: 10.1002/smll.201906707 [24] LI H, LI H X, DENG J F. Glucose hydrogenation over Ni-B/SiO2 amorphous alloy catalyst and the promoting effect of metal dopants[J]. Catal Today,2002,74(1/2):53−63. doi: 10.1016/S0920-5861(01)00530-2 [25] CHEN X Y, WANG S, ZHUANG J H, QIAO M H, FAN K N, HE H Y. Mesoporous silica-supported Ni-B amorphous alloy catalysts for selective hydrogenation of 2-ethylanthraquinone[J]. J Catal,2004,227(2):419−427. doi: 10.1016/j.jcat.2004.08.002 [26] SING K S W, EVERETT D H, HAUL R A W, MOSCOU L, PIEROTTI R A, ROUQUEROL J, SIEMIENIEWSKA T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity[J]. Pure Appl Chem,1985,57(4):603−619. doi: 10.1351/pac198557040603 [27] WANG W Y, YANG Y Q, LUO H A, PENG H Z, HE B, LIU W Y. Preparation of Ni(Co)-W-B amorphous catalysts for cyclopentanone hydrodeoxygenation[J]. Catal Commun,2011,12(14):1275−1279. doi: 10.1016/j.catcom.2011.04.027 [28] WANG S, HE P, XIE Z W, JIA L P, HE M Q, ZHANG X Q, DONG F Q, LIU H H, ZHANG Y, LI C X. Tunable nanocotton-like amorphous ternary Ni-Co-B: A highly efficient catalyst for enhanced oxygen evolution reaction[J]. Electrochim Acta,2019,296:644−652. doi: 10.1016/j.electacta.2018.11.099 [29] WANG Y Y, XIE C, ZHANG Z Y, LIU D D, CHEN R, WANG S Y. In situ exfoliated, N-doped, and edge-rich ultrathin layered double hydroxides nanosheets for oxygen evolution reaction[J]. Adv Funct Mater,2017,28(4):1703363. [30] WANG L N, LI Z, ZHANG P P, WANG G X, XIE G W. Hydrogen generation from alkaline NaBH4 solution using Co-Ni-Mo-P/γ-Al2O3 catalysts[J]. Int J Hydrogen Energy,2016,41(3):1468−1476. [31] WANG W Y, YANG S J, QIAO Z Q, LIU P L, WU K, YANG Y Q. Preparation of Ni-W-P-B amorphous catalyst for the hydrodeoxygenation of p-cresol[J]. Catal Commun,2015,60:50−54. doi: 10.1016/j.catcom.2014.11.023 [32] ZHANG Z, LIU Y D, HUANG Z Y, REN L, QI X, WEI X L, ZHONG J X. Facile hydrothermal synthesis of NiMoO4 @CoMoO4 hierarchical nanospheres for supercapacitor applications[J]. Phys Chem Chem Phys,2015,17(32):20795−20804. doi: 10.1039/C5CP03331D [33] PATEL N, FERNANDES R, MIOTELLO A. Hydrogen generation by hydrolysis of NaBH4 with efficient Co-P-B catalyst: A kinetic study[J]. J Power Sources,2009,188(2):411−420. doi: 10.1016/j.jpowsour.2008.11.121 [34] HU X J, SHI Y K, ZHANG Y J, ZHU B L, ZHANG S M, HUANG W P. Nanotubular TiO2-supported amorphous Co-B catalysts and their catalytic performances for hydroformylation of cyclohexene[J]. Catal Commun,2015,59:45−49. doi: 10.1016/j.catcom.2014.09.043 -





 下载:
下载: