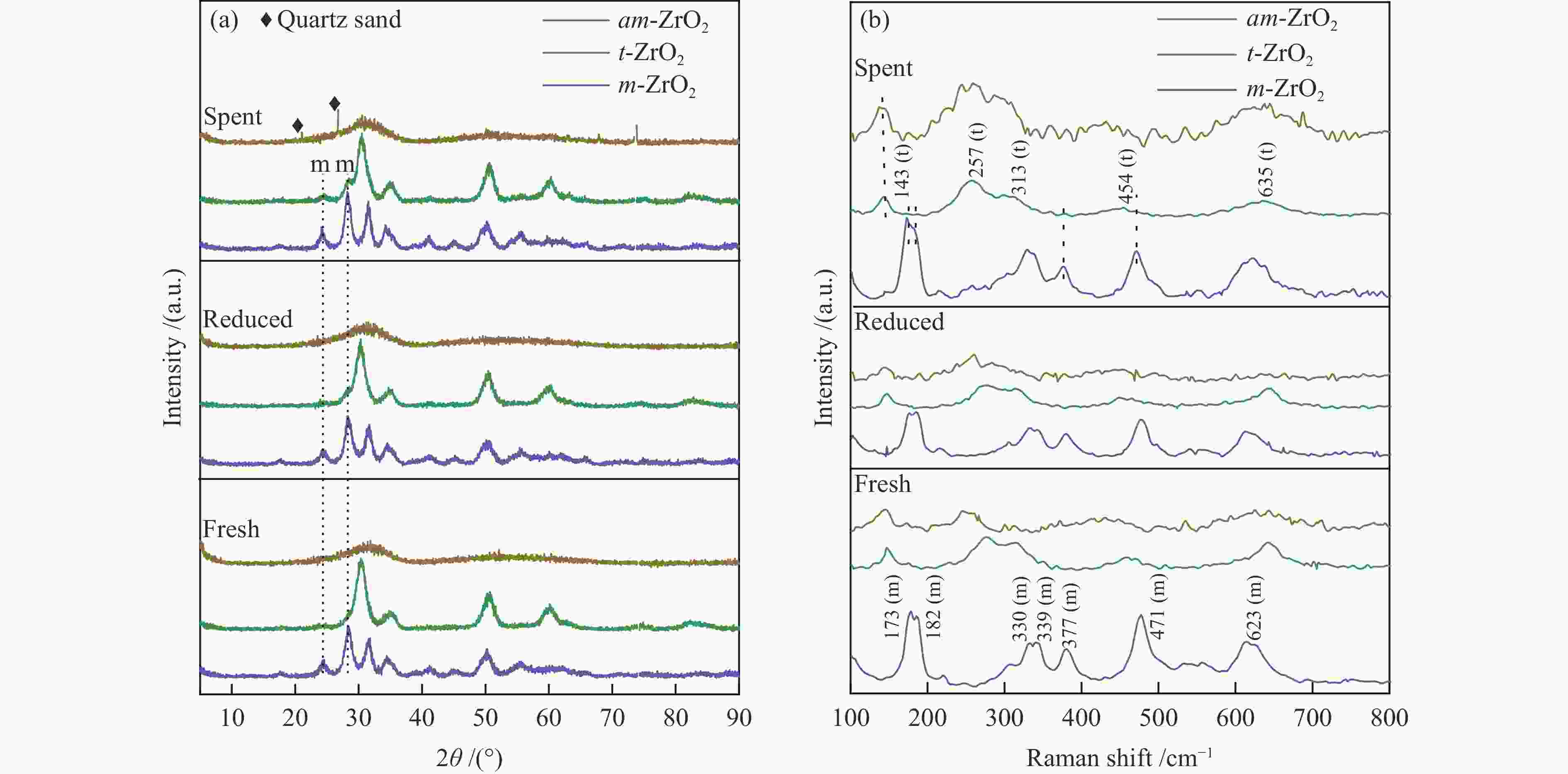
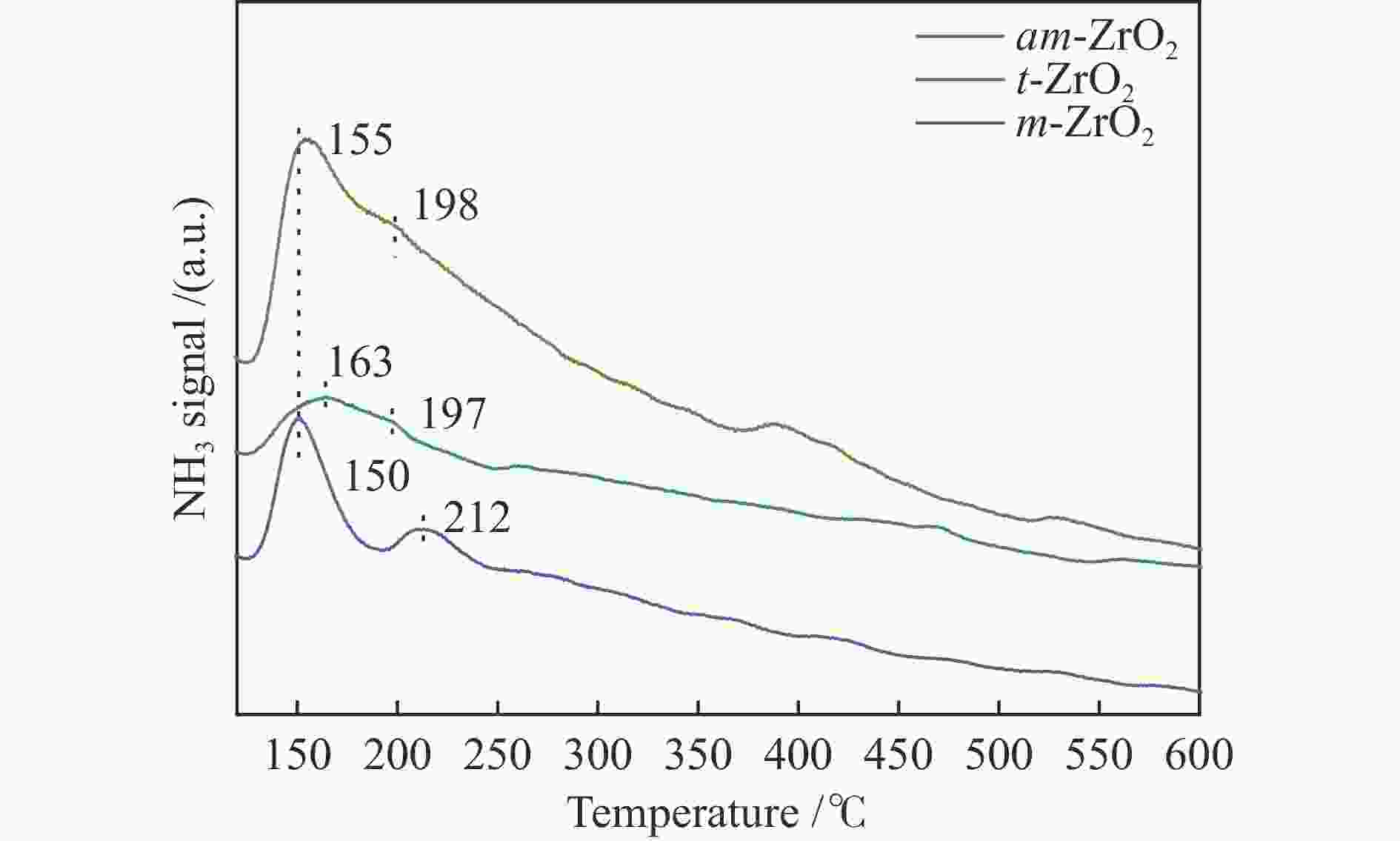
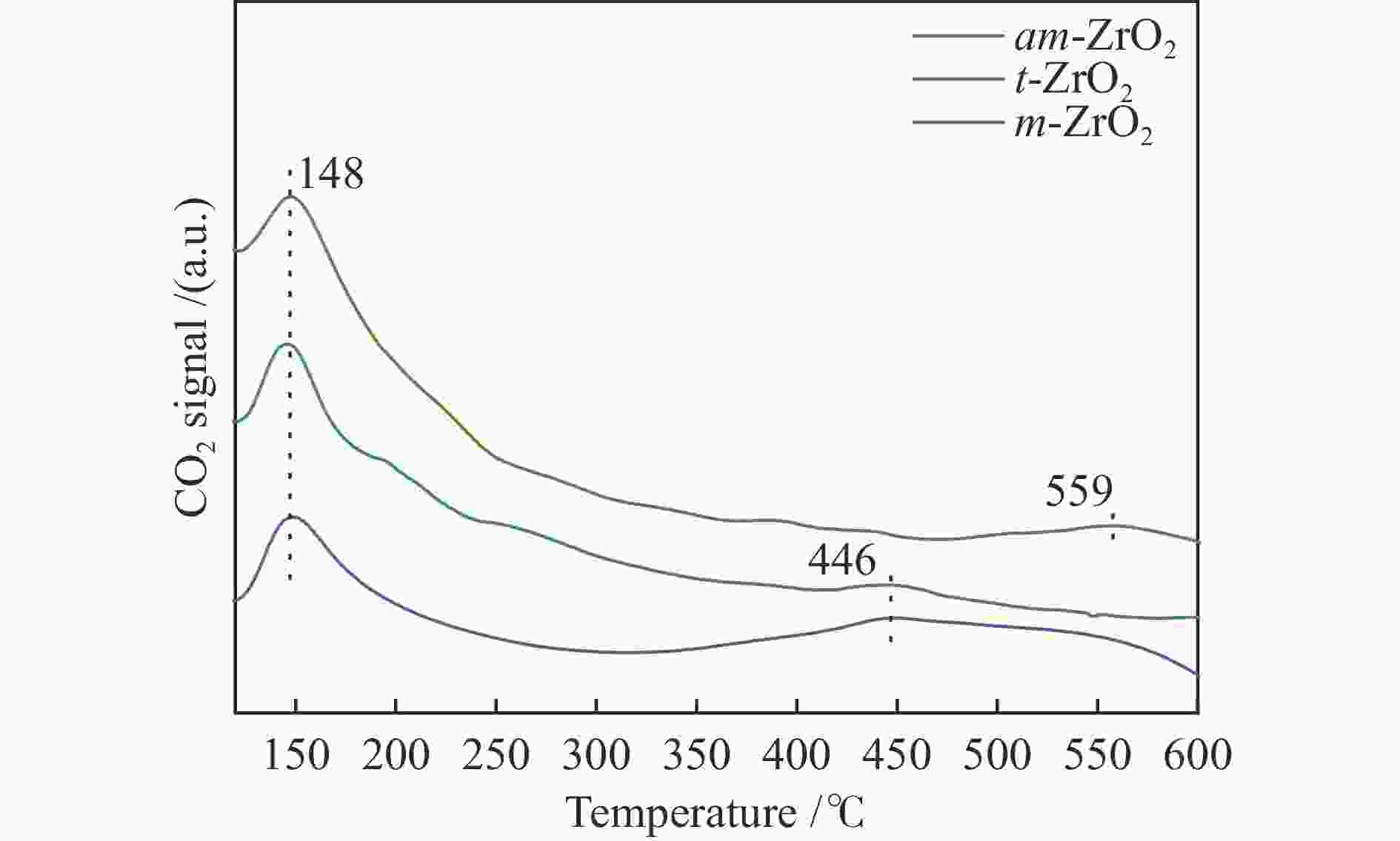
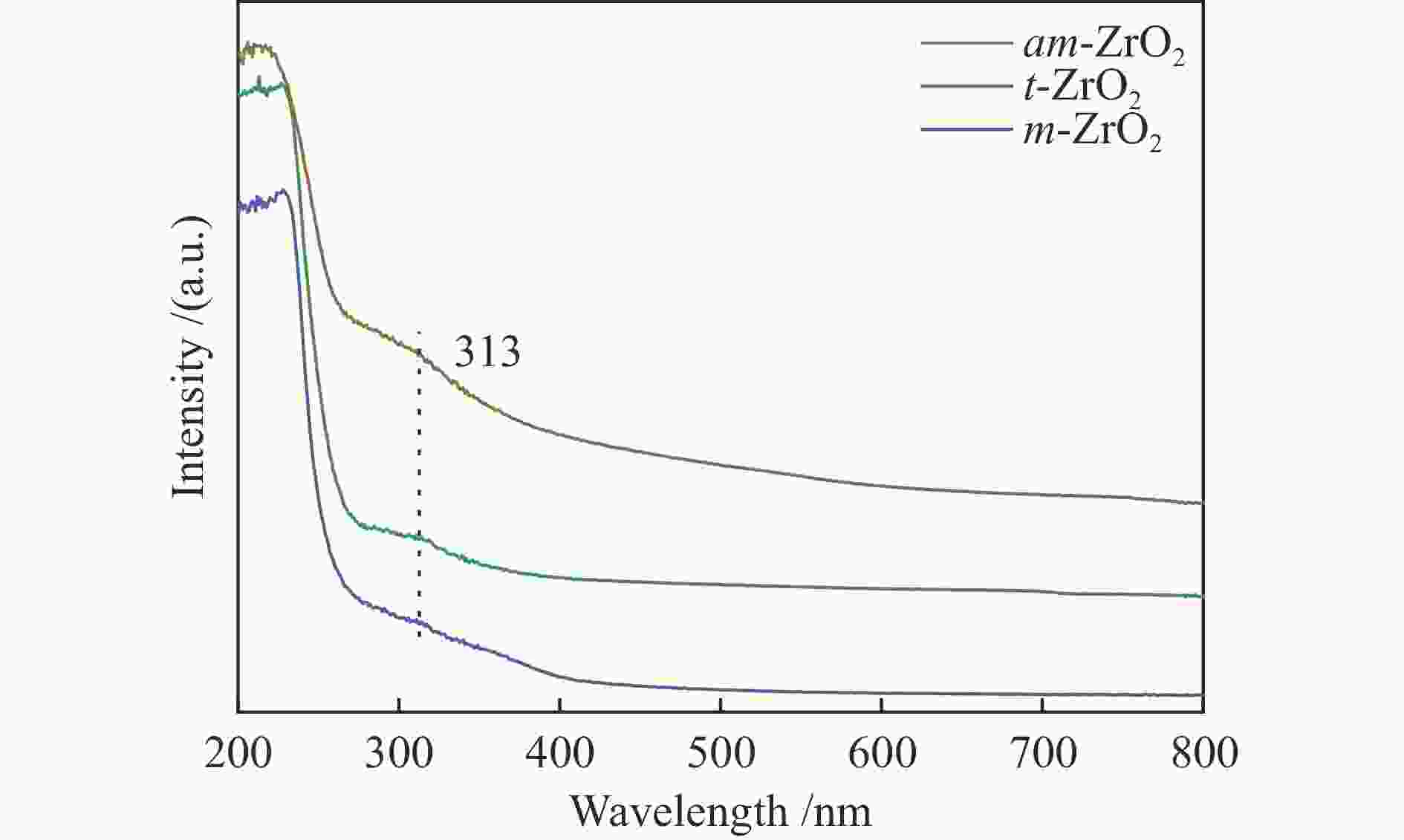
Effect of crystal structure of ZrO2 catalyst on isobutene synthesis from CO hydrogenation
-
摘要:
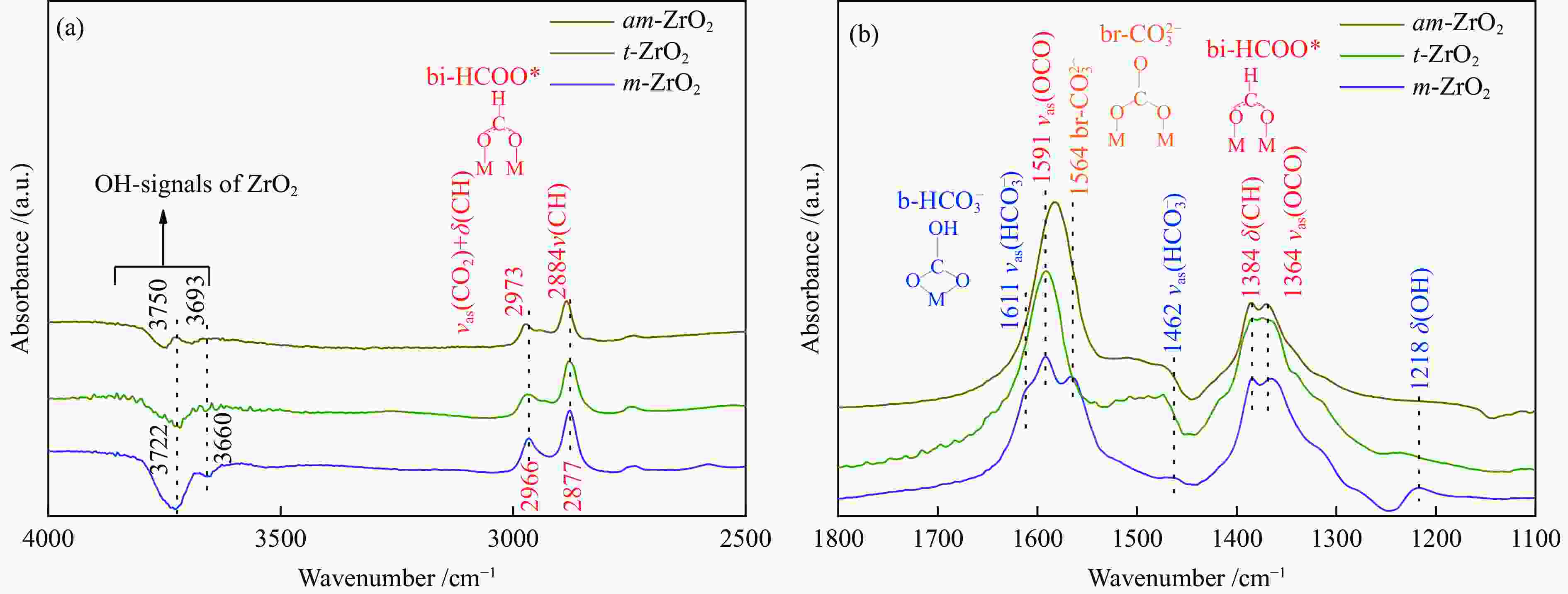
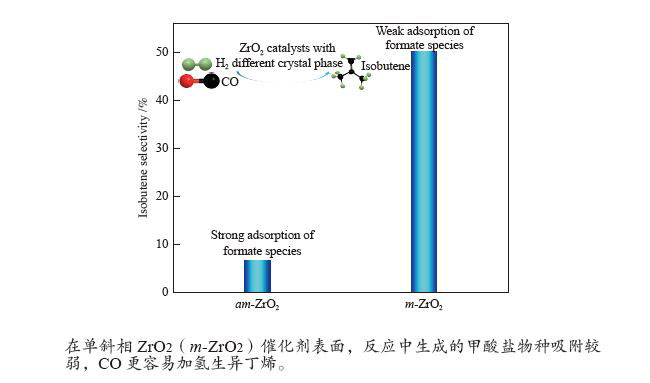
不同晶型结构的ZrO2在CO加氢制异丁烯反应中表现出不同的催化性能。尽管单斜相ZrO2在合成气制异丁烯反应中具有最优异的催化性能,但是对于其异构化活性位仍缺乏深入认识。通过研究ZrO2晶型结构对反应性能的影响差异,有利于深入认识ZrO2催化剂上合成气制异丁烯反应的关键影响因素。因此,本研究制备了一系列不同晶型结构的ZrO2催化剂,研究了它们在结构性质及催化CO加氢制异丁烯反应性能方面的差异。相对于四方相和无定型ZrO2,在单斜相ZrO2催化剂表面,有较多的配位不饱和的Zr位点和O位点。配位不饱和的Zr位点是CO吸附活化的位点,有利于CO的转化。而较多的不饱和配位的O位点,为异丁烯的生成提供了更多的碱性位。此外,在单斜相ZrO2催化剂表面,配位不饱和的Zr位点和O位点的存在,抑制了电子向反应中生成的甲酸盐物种转移,因此,甲酸盐物种在催化剂表面吸附较弱,有利于CO加氢生成异丁烯。
Abstract:ZrO2 catalysts with different crystal structures show different catalytic performance in isobutene synthesis from CO hydrogenation reaction. Although monoclinic ZrO2 has the best catalytic performance in isobutene synthesis from syngas, its isosynthesis active sites are still not well understood. To better understand the critical parameters that influence syngas to isobutene reactions over ZrO2 catalysts, we prepared a series of ZrO2 catalysts with distinct crystal structures and investigated their catalytic performance of CO hydrogenation to isobutene. Compared with tetragonal and amorphous ZrO2 catalysts, there are more coordinatively unsaturated Zr and O sites on the surface of monoclinic ZrO2 catalyst. The coordinatively unsaturated Zr sites are the active sites of CO adsorption and activation, which is beneficial to CO conversion. The coordinatively unsaturated O sites provide more basic sites for isobutene formation. Furthermore, the coordinatively unsaturated Zr and O sites on monoclinic ZrO2 catalyst surface may inhibit electron transfer to formate species formed during reaction, resulting in weak adsorption on catalyst surface of formate species. The weakly adsorbed formate species on the surface of monoclinic ZrO2 catalyst is favorable for the synthesis of isobutene from CO hydrogenation reaction.
-
Key words:
- CO hydrogenation /
- isobutene /
- ZrO2 /
- crystal structures
-
表 1 不同晶型结构ZrO2催化剂上CO加氢制异丁烯催化性能a
Table 1 Catalytic performance of CO hydrogenation to isobutene over ZrO2 catalysts with different crystal phase a
Catalyst CO Conv.
/%bSel. Cmol/% Products distribution in CHn CHnb DME CO2 C1 ${\rm{C} }_{{2}}^=$ ${\rm{C}}_{{3}}^= $ ${ {n} }{\text{-} }{\rm{C} }_{{4} }^{= \;{\rm{c} } }$ ${ {i} }{\text{-} }{\rm{C} }_{{4} }^{= \;{\rm{c} } }$ C5 + m-ZrO2 31.9 32.1 3.9 64.0 3.3 8.6 (1.7) 3.0 (2.5) 3.6 (2.1)d 50.2 25.0 t-ZrO2 24.7 36.1 6.7 57.2 14.9 5.1 (5.0) 2.0 (2.7) 6.7 (3.1) 38.4 22.1 am-ZrO2 31.1 35.1 0.0 64.9 32.5 6.6 (12.4) 6.1 (4.7) 9.3 (7.9) 6.5 14.0 a: Reaction conditions: H2/CO=1, 400 ℃, 5.0 MPa, GHSV=430 h−1, catalysts were pretreated in a stream of H2/N2=1∶10 at 400 ℃ for 6 h before reaction;
b: CHn means the hydrocarbons products except for oxygenated chemicals and CO2;
c: ${{n}}{\text{-}}{\rm{C}}_{{4}}^{=} $ and ${{i}}{\text{-}}{\rm{C}}_{{4}}^{=} $ stand for the linear butenes and isobutene, respectively;
d: data in parentheses mean the selectivity of alkane (Cn) in total hydrocarbons表 2 不同晶型结构ZrO2样品的织构性质参数
Table 2 Textural properties of the resulting ZrO2 samples
Sample SBET/(m2∙g−1) a vpore/(cm3∙g−1) b dave/nm c m-ZrO2 125.6 0.26 8.2 t-ZrO2 85.8 0.09 4.3 am-ZrO2 215.0 0.16 3.1 a: BJH desorption cumulative surface area of pores between 1.7 and 300 nm width;
b: BJH desorption cumulative volume of pores between 1.7 and 300 nm width;
c: BJH desorption average pore width (4V/A)表 3 ZrO2样品的XPS拟合
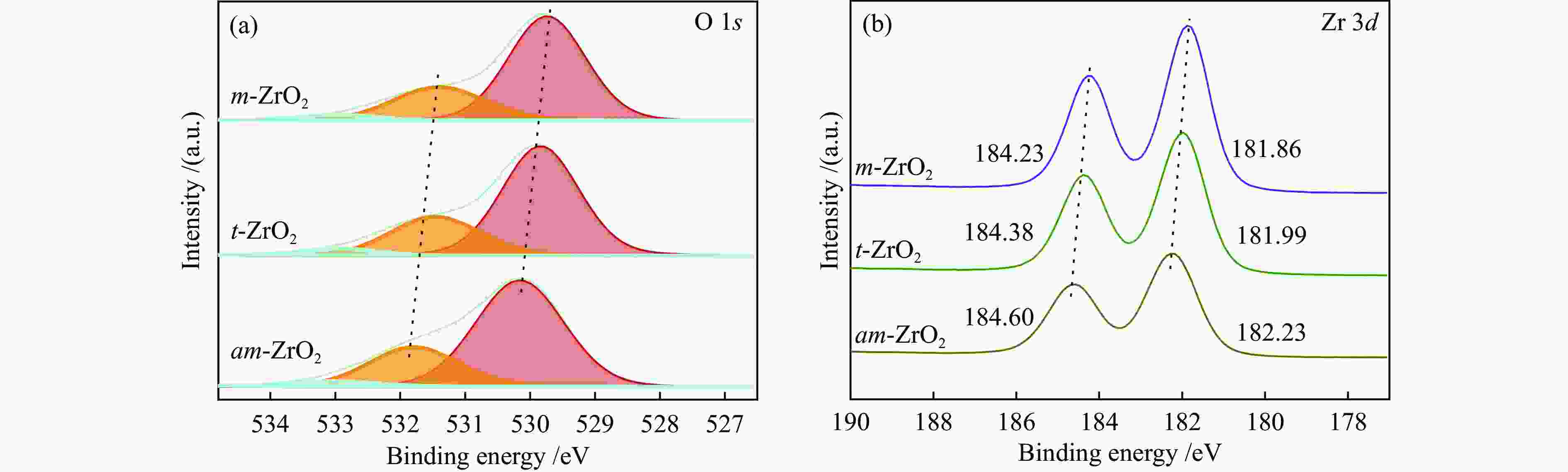
Table 3 XPS fitting results of ZrO2 samples
Sample Binding energy /eV Area/% Zr 3d5/2 Zr 3d3/2 OLattice ODefect OOH OLattice ODefect OOH OLattice/
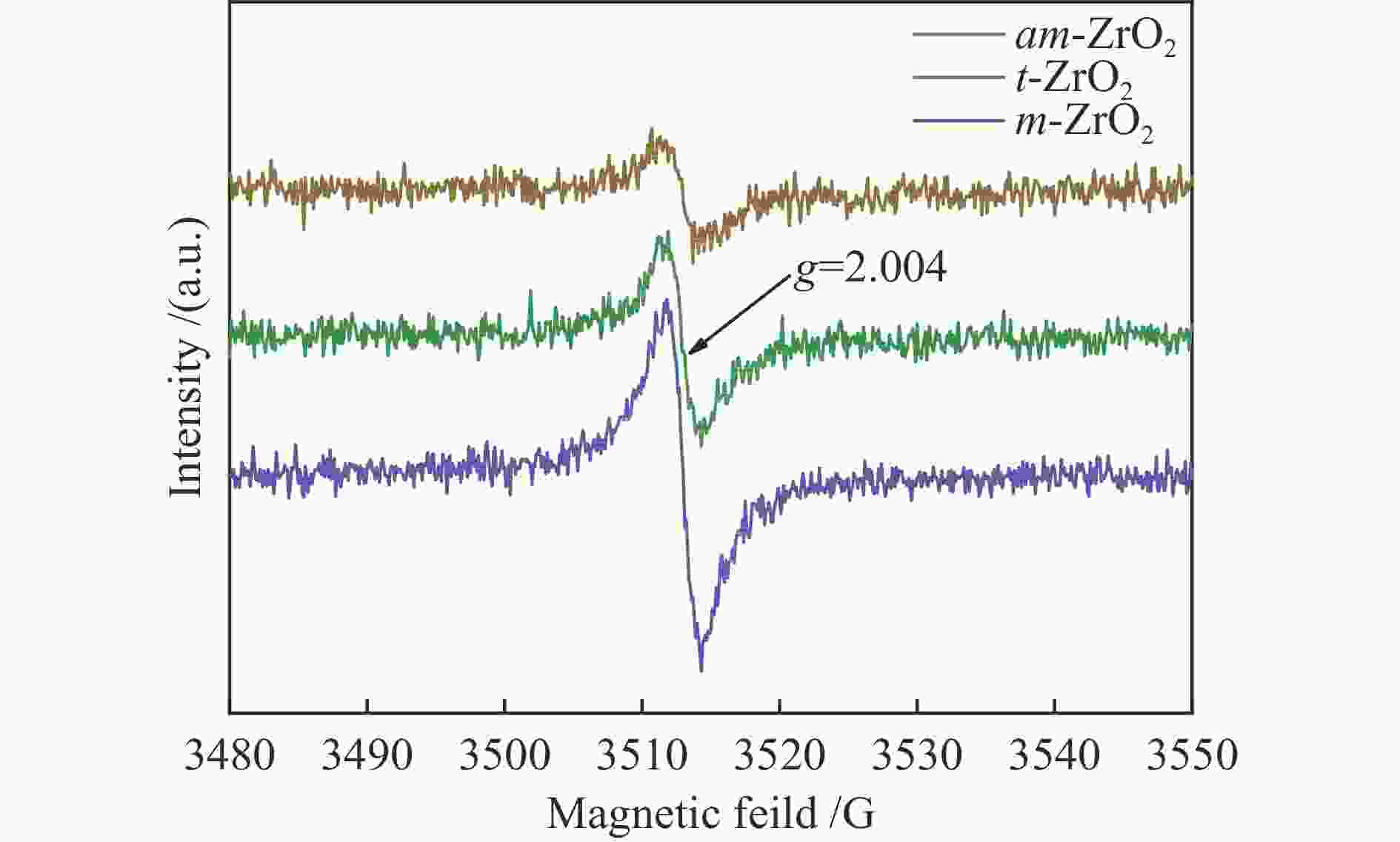
(ODefect + OOH)m-ZrO2 181.86 184.23 529.74 531.40 532.94 70.8 25.7 3.6 0.41 t-ZrO2 181.99 184.38 529.84 531.46 532.94 69.1 26.5 4.4 0.45 am-ZrO2 182.23 184.60 530.16 531.79 533.00 70.0 26.1 3.9 0.43 -
[1] ZHANG R, LIU H, HE D. Pure monoclinic ZrO2 prepared by hydrothermal method for isosynthesis[J]. Catal Commun,2012,26:244−247. doi: 10.1016/j.catcom.2012.06.005 [2] WELLS A F. Structural Inorganic Chemistry[M]. Oxford: Oxford University Press, 2012. [3] LOONG C K, RICHARDSON J W, OZAWA M. Crystal phases, defects, and dynamics of adsorbed hydroxyl groups and water in pure and lanthanide-modified zirconia: A neutron-scattering study[J]. J Catal,1995,157(2):636−644. doi: 10.1006/jcat.1995.1329 [4] NOBUTA Y, TAKAHASHI Y, MIYAZAKI T, TERAKADO N, ONOUE N, SHINOZAKI T, FUJIWARA T. Crystallization of nanostructured ZrO2 phase in borosilicate glass: Impact of Al2O3 on tetragonal-to-monoclinic phase transformation[J]. J Non-Cryst Solids,2018,501:49−54. doi: 10.1016/j.jnoncrysol.2017.12.052 [5] BUMAJDAD A, NAZEER A A, SAGHEER F A, NAHAR S, ZAKI M I. Controlled synthesis of ZrO2 nanoparticles with tailored size, morphology and crystal phases via organic/inorganic hybrid films[J]. Sci Rep,2018,8(1):1−9. [6] SATO A, VOLANTI D, MEIRA D, DAMYANOVA S, LONGO E, BUENO J M C. Effect of the ZrO2 phase on the structure and behavior of supported Cu catalysts for ethanol conversion[J]. J Catal,2013,307:1−17. doi: 10.1016/j.jcat.2013.06.022 [7] 李文, 张文忠, 殷元骐. 三种晶型ZrO2的制备及其催化性能的研究[J]. 天然气化工,1995,20(2):1−3.LI Wen, ZHANG Wen-zhong, YIN Yuan-qi. Preparation of three crystal shapes of ZrO2 and their catalytic performances[J]. Nat Gas Chem Ind,1995,20(2):1−3. [8] 李文, 殷元骐, 高润雄, 侯瑞玲. 单斜及四方晶相ZrO2催化CO加氢反应性能的比较[J]. 分子催化,1999,13(3):186−192.LI Wen, YIN Yuan-qi, GAO Run-xiong, HOU Rui-ling. Comparison of CO hydrogenation on monoclinic and tetragonal ZrO2 catalysts[J]. J Mol Catal,1999,13(3):186−192. [9] LU L, HAYAKAWA T, UEDA T, HARA M, DOMEN K, MARUYA K, YASHIMA M. Dependence of catalytic activity in CO hydrogenation on strong basic sites of ZrO2 surface[J]. Chem Lett,1998,27(1):65−66. doi: 10.1246/cl.1998.65 [10] MARUYA K, KOMIYA T, OKUMURA K, YASHIMA M. Linear relationship of the rate of isobutene formation from CO and H2 on ZrO2 to the monoclinic phase fraction[J]. Chem Lett,1999,28(7):575−576. doi: 10.1246/cl.1999.575 [11] JACKSON N B, EKERDT J G. The surface characteristics required for isosynthesis over zirconium dioxide and modified zirconium dioxide[J]. J Catal,1990,126(1):31−45. doi: 10.1016/0021-9517(90)90044-K [12] LI Y, HE D, YUAN Y, CHEN Z, ZHU Q. Influence of acidic and basic properties of ZrO2 based catalysts on isosynthesis[J]. Fuel,2002,81(11):1611−1617. [13] LI Y, HE D, ZHU Q, ZHANG X, XU B. Effects of redox properties and acid-base properties on isosynthesis over ZrO2-based catalysts[J]. J Catal,2004,221(2):584−593. doi: 10.1016/j.jcat.2003.09.023 [14] LI Y, HE D, CHENG Z, SU C, LI J, ZHU Q. Effect of calcium salts on isosynthesis over ZrO2 catalysts[J]. J Mol Catal A: Chem,2001,175(1):267−275. [15] WU X, TAN M, XU B, ZHAO S, MA Q, HE Y, ZENG C, YANG G, TSUBAKI N, TAN Y. Tuning the crystallite size of monoclinic ZrO2 to reveal critical roles of surface defects on m-ZrO2 catalyst for direct synthesis of isobutene from syngas[J]. Chin J Chem Eng,2021,35:211−219. doi: 10.1016/j.cjche.2021.04.008 [16] CHATTERJEE A, PRADHAN S, DATTA A, DE M, CHAKRAVORTY D. Stability of cubic phase in nanocrystalline ZrO2[J]. J Mater Res,1994,9(2):263−265. doi: 10.1557/JMR.1994.0263 [17] KIM D, TIEN T. Phase stability and physical properties of cubic and tetragonal ZrO2 in the system ZrO2-Y2O3-Ta2O5[J]. J Am Ceram Soc,1991,74(12):3061−3065. doi: 10.1111/j.1151-2916.1991.tb04302.x [18] LI W, HUANG H, LI H, ZHANG W, LIU H. Facile synthesis of pure monoclinic and tetragonal zirconia nanoparticles and their phase effects on the behavior of supported molybdena catalysts for methanol-selective oxidation[J]. Langmuir,2008,24(15):8358−8366. doi: 10.1021/la800370r [19] REZAEI M, ALAVI S, SAHEBDELFAR S, BAI P, LIU X, YAN Z. CO2 reforming of CH4 over nanocrystalline zirconia-supported nickel catalysts[J]. Appl Catal B: Environ,2008,77(3/4):346−354. doi: 10.1016/j.apcatb.2007.08.004 [20] 李美俊. 氧化锆和掺杂氧化锆物相及其变化的紫外拉曼光谱研究[D]. 大连: 中国科学院大连化学物理研究所, 2002.LI Mei-jun. UV Raman spectroscopic studies on the phase structure and phase transformation of zirconia and doped zirconia[D]. Dalian: Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 2002. [21] YI M, ZHANG Y, XU J, DENG D, MAO Z, MENG X, SHI X, ZHAO B. Surface-enhanced Raman scattering activity of ZrO2 nanoparticles: Effect of tetragonal and monoclinic phases[J]. Nanomaterials,2021,11(9):2162. doi: 10.3390/nano11092162 [22] ZHAO X, VANDERBILT D. Phonons and lattice dielectric properties of zirconia[J]. Phys Rev B,2002,65(7):075105. doi: 10.1103/PhysRevB.65.075105 [23] BACHILLER-BAEZA B, RODRIGUEZ-RAMOS I, GUERRERO-RUIZ A. Interaction of carbon dioxide with the surface of zirconia polymorphs[J]. Langmuir,1998,14(13):3556−3564. doi: 10.1021/la970856q [24] RASHAD M, BAIOUMY H. Effect of thermal treatment on the crystal structure and morphology of zirconia nanopowders produced by three different routes[J]. J Mater Process Technol,2008,195(1/3):178−185. doi: 10.1016/j.jmatprotec.2007.04.135 [25] BOTTA S, NAVÍO J, HIDALGO M, RESTREPO G, LITTER M. Photocatalytic properties of ZrO2 and Fe/ZrO2 semiconductors prepared by a sol-gel technique[J]. J Photoch Photobio A,1999,129(1/2):89−99. doi: 10.1016/S1010-6030(99)00150-1 [26] KUMARI L, LI W, XU J, LEBLANC R, WANG D, LI Y, GUO H, ZHANG J. Controlled hydrothermal synthesis of zirconium oxide nanostructures and their optical properties[J]. Cryst Growth Des,2009,9(9):3874−3880. doi: 10.1021/cg800711m [27] GIONCO C, PAGANINI M, GIAMELLO E, VALENTIN C, PACCHIONI G. Paramagnetic defects in polycrystalline zirconia: An EPR and DFT study[J]. Chem Mater,2013,25(11):2243−2253. doi: 10.1021/cm400728j [28] IVANOVSKAYA M, FROLOVA E. Nature and conditions of formation of structural defects in zirconium (IV) oxide in the course of its preparation from zirconium hydroxide[J]. Russ J Gen Chem,2007,77(4):524−531. doi: 10.1134/S1070363207040056 [29] DANG S, GAO P, LIU Z, CHEN X, YANG C, WANG H, ZHONG L, LI S, SUN Y. Role of zirconium in direct CO2 hydrogenation to lower olefins on oxide/zeolite bifunctional catalysts[J]. J Catal,2018,364:382−393. doi: 10.1016/j.jcat.2018.06.010 [30] DUPIN J, GONBEAU D, VINATIER P, LEVASSEUR A. Systematic XPS studies of metal oxides, hydroxides and peroxides[J]. PCCP,2000,2(6):1319−1324. doi: 10.1039/a908800h [31] FAN H, YANG S, ZHANG P, WEI H, LIU X, JIAO C, ZHU Q, CHEN Y, WANG Z. Investigation of oxygen vacancy and interstitial oxygen defects in ZnO films by photoluminescence and X-ray photoelectron spectroscopy[J]. Chin Phys Lett,2007,24(7):2108−2111. doi: 10.1088/0256-307X/24/7/089 [32] ZHANG M, ZHANG J, WU Y, PAN J, ZHANG Q, TAN Y, HAN Y. Insight into the effects of the oxygen species over Ni/ZrO2 catalyst surface on methane reforming with carbon dioxide[J]. Appl Catal B: Environ,2019,244:427−437. doi: 10.1016/j.apcatb.2018.11.068 [33] MENG C, ZHAO G, SHI X, CHEN P, LIU Y, LIU Y. Oxygen-deficient metal oxides supported nano-intermetallic InNi3C0.5 toward efficient CO2 hydrogenation to methanol[J]. Sci Adv,2021,7(32):eabi6012. doi: 10.1126/sciadv.abi6012 [34] POKROVSKI K, JUNG K T, BELL A T. Investigation of CO and CO2 adsorption on tetragonal and monoclinic zirconia[J]. Langmuir,2001,17(14):4297−4303. doi: 10.1021/la001723z [35] KÖCK E M, KOGLER M, BIELZ T, KLÖTZER B, PENNERM. In situ FT-IR spectroscopic study of CO2 and CO adsorption on Y2O3, ZrO2, and yttria-stabilized ZrO2[J]. J Phys Chem C,2013,117(34):17666−17673. doi: 10.1021/jp405625x [36] KORHONEN S, CALATAYUD M, KRAUSE A O. Structure and stability of formates and carbonates on monoclinic zirconia: A combined study by density functional theory and infrared spectroscopy[J]. J Phys Chem C,2008,112(41):16096−16102. doi: 10.1021/jp803353v [37] RHODES M, BELL A. The effects of zirconia morphology on methanol synthesis from CO and H2 over Cu/ZrO2 catalysts: Part I. Steady-state studies[J]. J Catal,2005,233(1):198−209. doi: 10.1016/j.jcat.2005.04.026 [38] HE M, EKERDT J. Infrared studies of the adsorption of synthesis gas on zirconium dioxide[J]. J Catal,1984,87(2):381−388. doi: 10.1016/0021-9517(84)90198-2 [39] YANG R, FU Y, ZHANG Y, TSUBAKI N. In situ DRIFT study of low-temperature methanol synthesis mechanism on Cu/ZnO catalysts from CO2-containing syngas using ethanol promoter[J]. J Catal,2004,228(1):23−35. doi: 10.1016/j.jcat.2004.08.017 [40] OUYANG F, NAKAYAMA A, TABADA K, SUZUKI E. Infrared study of a novel acid-base site on ZrO2 by adsorbed probe molecules. I. Pyridine, carbon dioxide, and formic acid adsorption[J]. J Phys Chem B,2000,104(9):2012−2018. doi: 10.1021/jp992970i [41] HERTL W. Surface chemistry of zirconia polymorphs[J]. Langmuir,1989,5(1):96−100. doi: 10.1021/la00085a018 [42] WU X, TAN M, TIAN S, SONG F, MA Q, HE Y, YANG G, TSUBAKI N, TAN Y. Designing ZrO2-based catalysts for the direct synthesis of isobutene from syngas: The studies on Zn promoter role[J]. Fuel,2019,243:34−40. doi: 10.1016/j.fuel.2019.01.098 [43] MA S, HUANG S, LIU Z. Dynamic coordination of cations and catalytic selectivity on zinc-chromium oxide alloys during syngas conversion[J]. Nat Catal,2019,2:671−677. doi: 10.1038/s41929-019-0293-8 [44] 相宏伟, 钟炳, 彭少逸. CO + H2 在 ZrO2 催化剂上合成异构烃反应机理的研究[J]. 天然气化工: C1 化学与化工,1994,19(4):14−19.XIANG Hong-wei, ZHONG Bing, PENG Shao-yi. Study on isohydrocarbon reaction mechanism from CO + H2 over ZrO2 catalyst[J]. Nat Gas Chem Ind,1994,19(4):14−19. [45] JACKSON N B, EKERDT J G. Isotope studies of the effect of acid sites on the reactions of C3 intermediates during isosynthesis over zirconium dioxide and modified zirconium dioxide[J]. J Catal,1990,126(1):46−56. doi: 10.1016/0021-9517(90)90045-L -




 下载:
下载: