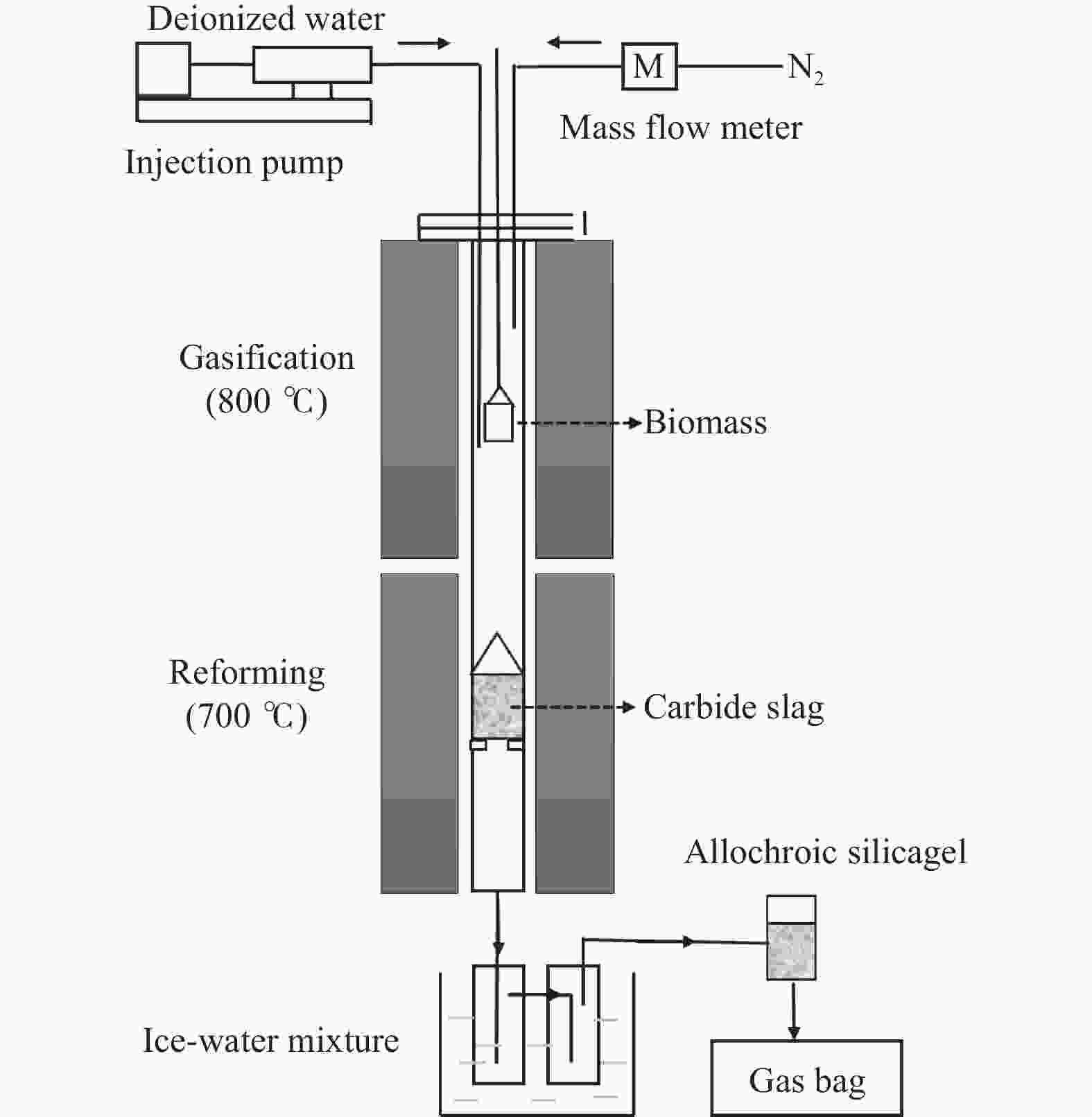
Study of calcium-based waste on adsorption enhanced biomass gasification for hydrogen production
-
摘要:
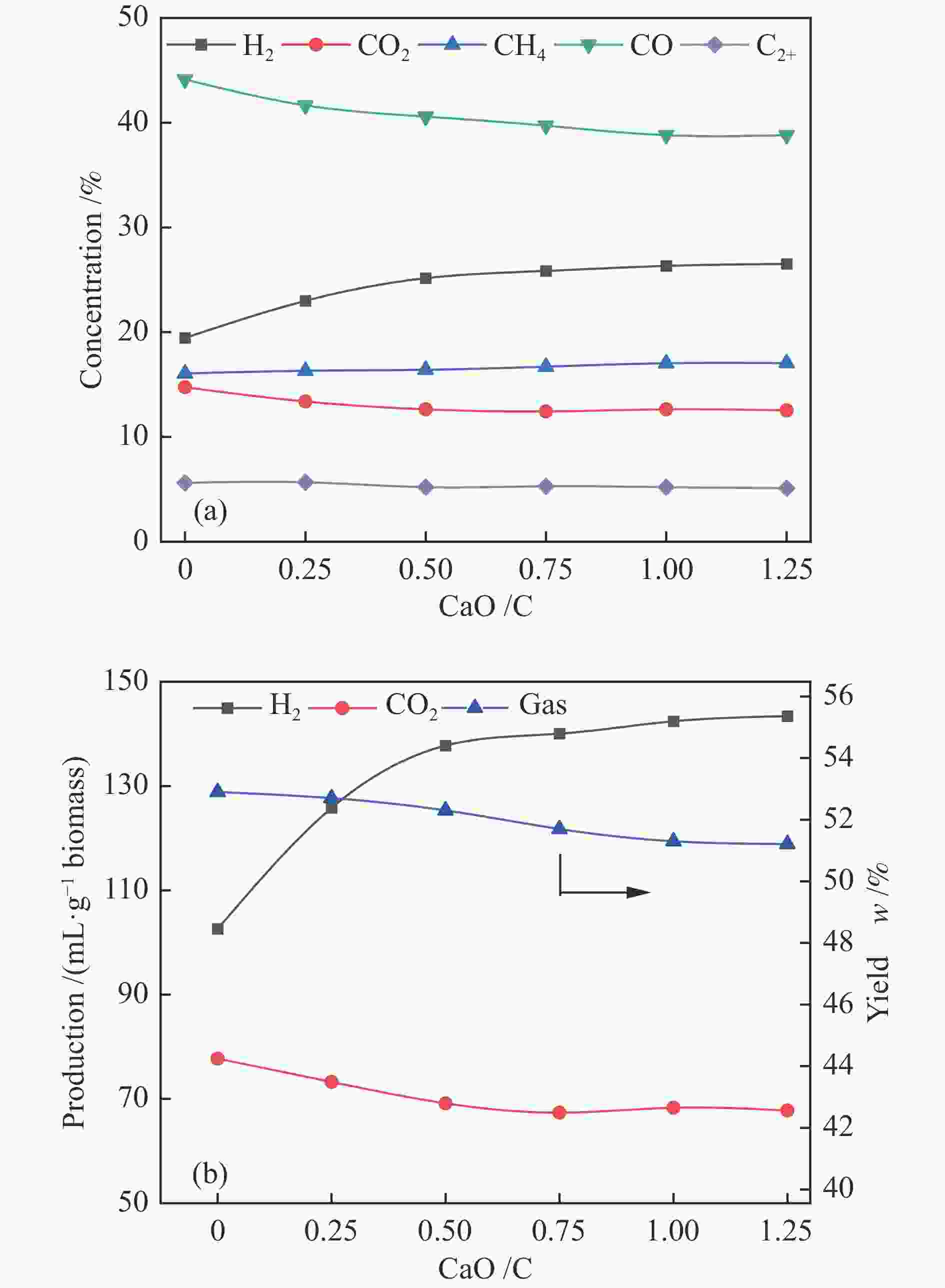
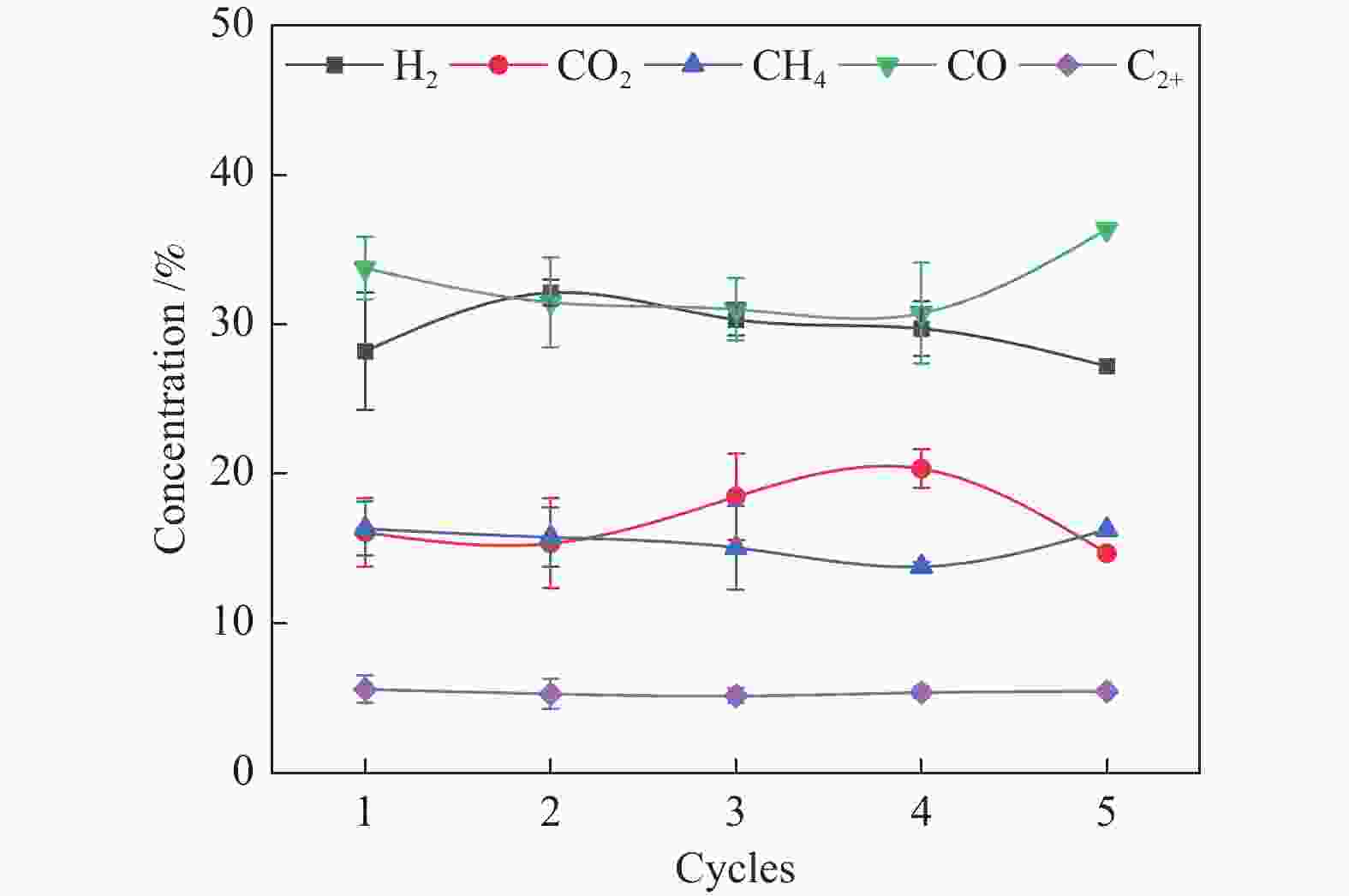
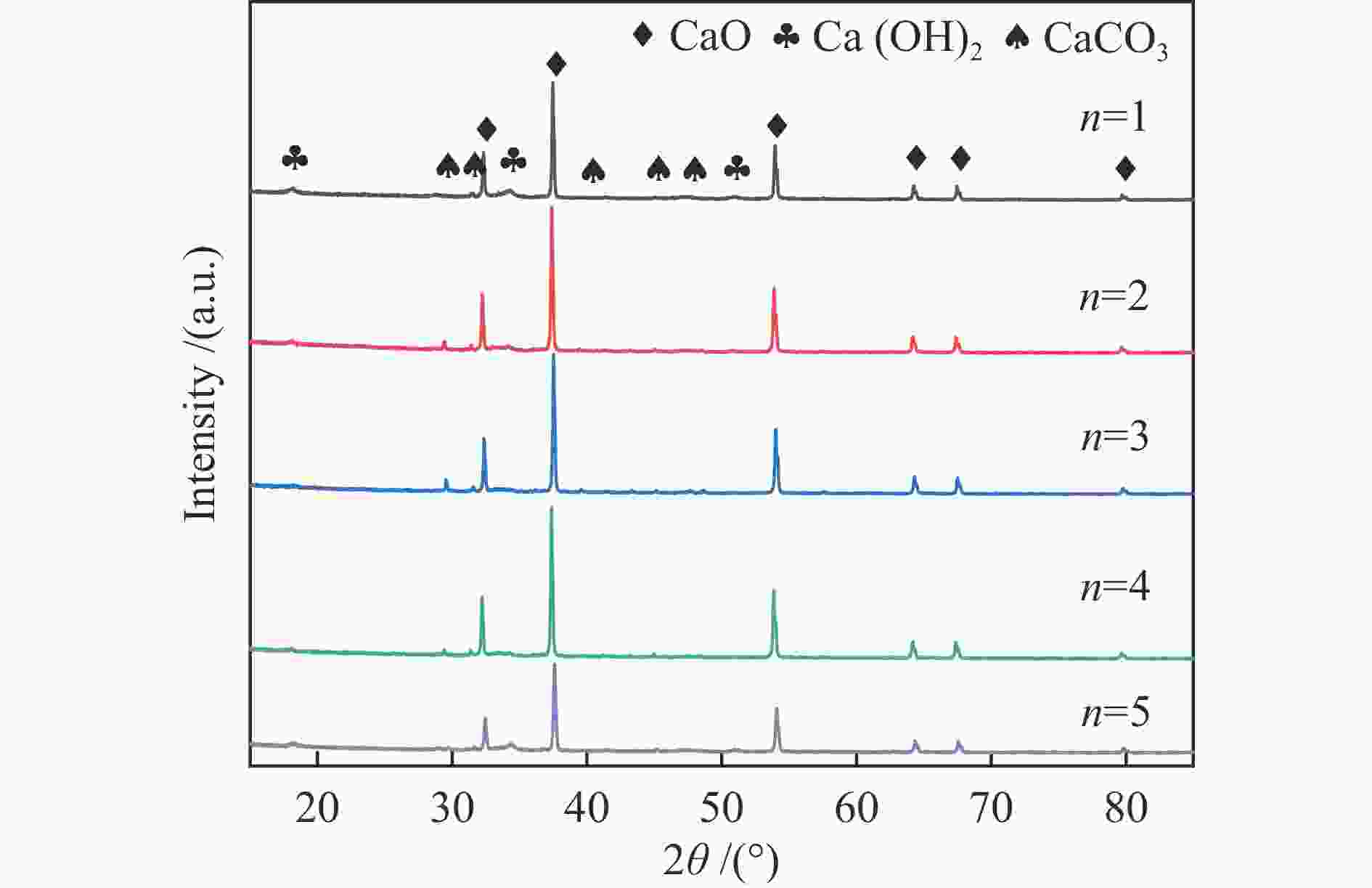
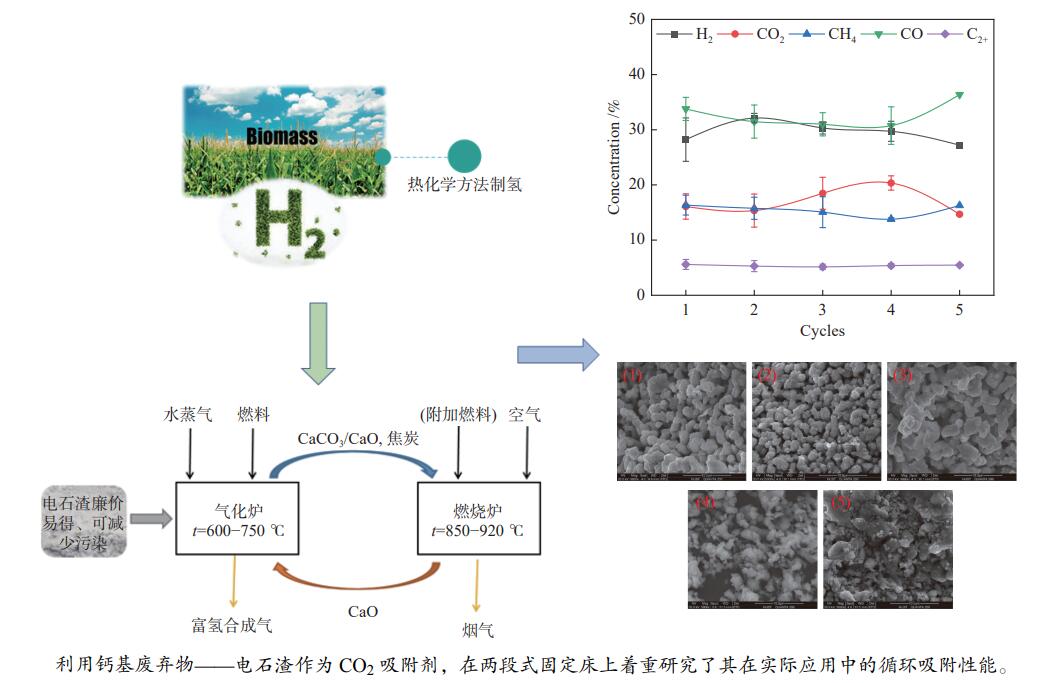
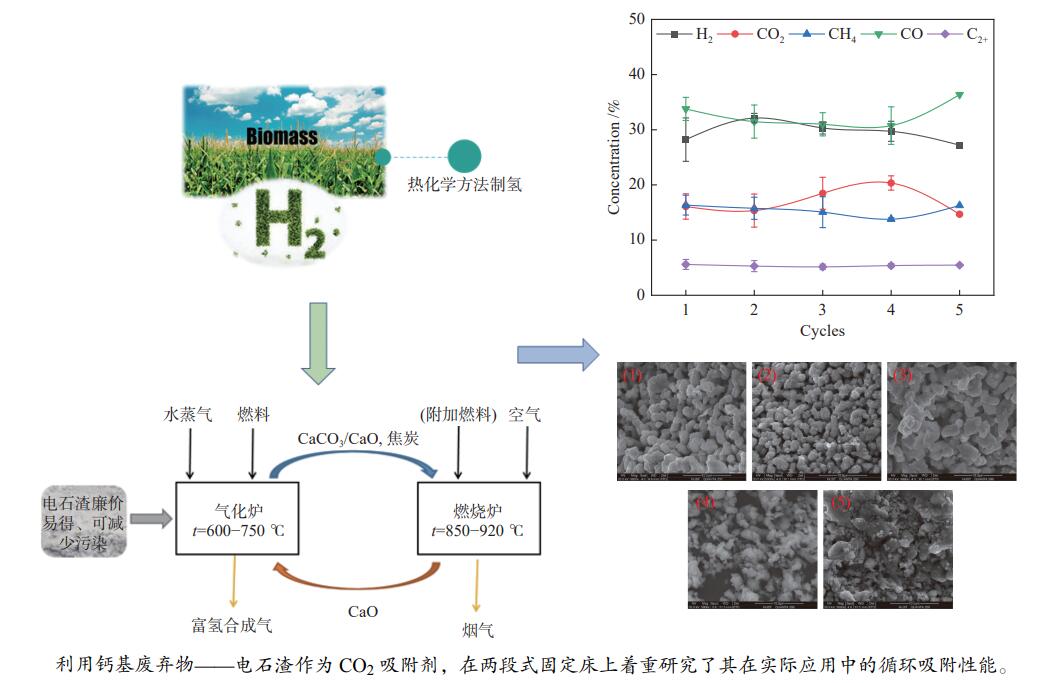
为了提高生物质气化制氢效率,综合利用工业固废资源,利用钙基废弃物——电石渣作为CO2吸附剂,在两段式固定床上探究了钙基废弃物的添加量、反应温度对生物质气化制氢特性的影响,着重研究了吸附剂在实际应用中的循环吸附性能,并以此探讨了电石渣对生物质吸附增强气化的影响机制。结果表明,随着电石渣添加量的逐渐增加,H2产量和含量都呈现出增加的趋势。而随着温度的升高,H2产量和含量先增加后减小。当CaO/C物质的量比为1,重整段温度为700 ℃时,气体产物中的H2产量和含量为154.34 mL/g(生物质)和26.76%,获得最大值。当电石渣循环次数小于5时,H2的含量和产量相较于初次反应都有所增加。
Abstract:In order to improve the efficiency of biomass gasification to hydrogen production, the comprehensive utilization of industrial solid waste resources, the use of calcium-based waste-calcium carbide slag as a CO2 adsorbent, experiments were carried out in a two-stage fixed bed to explore the effect of calcium-based waste addition, reaction temperature on the biomass gasification hydrogen production characteristics, focusing on the study of the adsorbent in practical applications of the cyclic adsorption performance, and thus discuss the influence mechanism of calcium carbide slag on biomass adsorption enhanced gasification. The results show that with the gradual increase of calcium carbide slag addition, H2 yield and concentration show an increasing trend. With the increase of temperature, the yield and concentration of H2 increase first and then decrease. When the CaO/C molar ratio is 1 and the temperature of the reforming section is 700 ℃, the yield and concentration of H2 in the gas product are 154.34 mL/g biomass and 26.76%, and the maximum value is obtained. When the number of calcium carbide slag cycles is less than 5, the concentration and yield of H2 increase compared to the initial reaction.
-
表 1 生物质样品的工业分析和元素分析
Table 1 Proximate and ultimate analyses of samples
Sample Proximate analysis wd/% Ultimate analysis wd/% QHHV/(MJ·kg−1) O/C H/C A FC V C H O* N Bamboo shavings 5.47 7.75 86.78 53.32 6.64 34.21 0.2 21.62 0.48 1.49 note:FC -fixed carbon; V-volatile; A-ash; d-dry basis; * - by difference 表 2 电石渣XRF成分分析
Table 2 XRF composition analysis of carbide slag
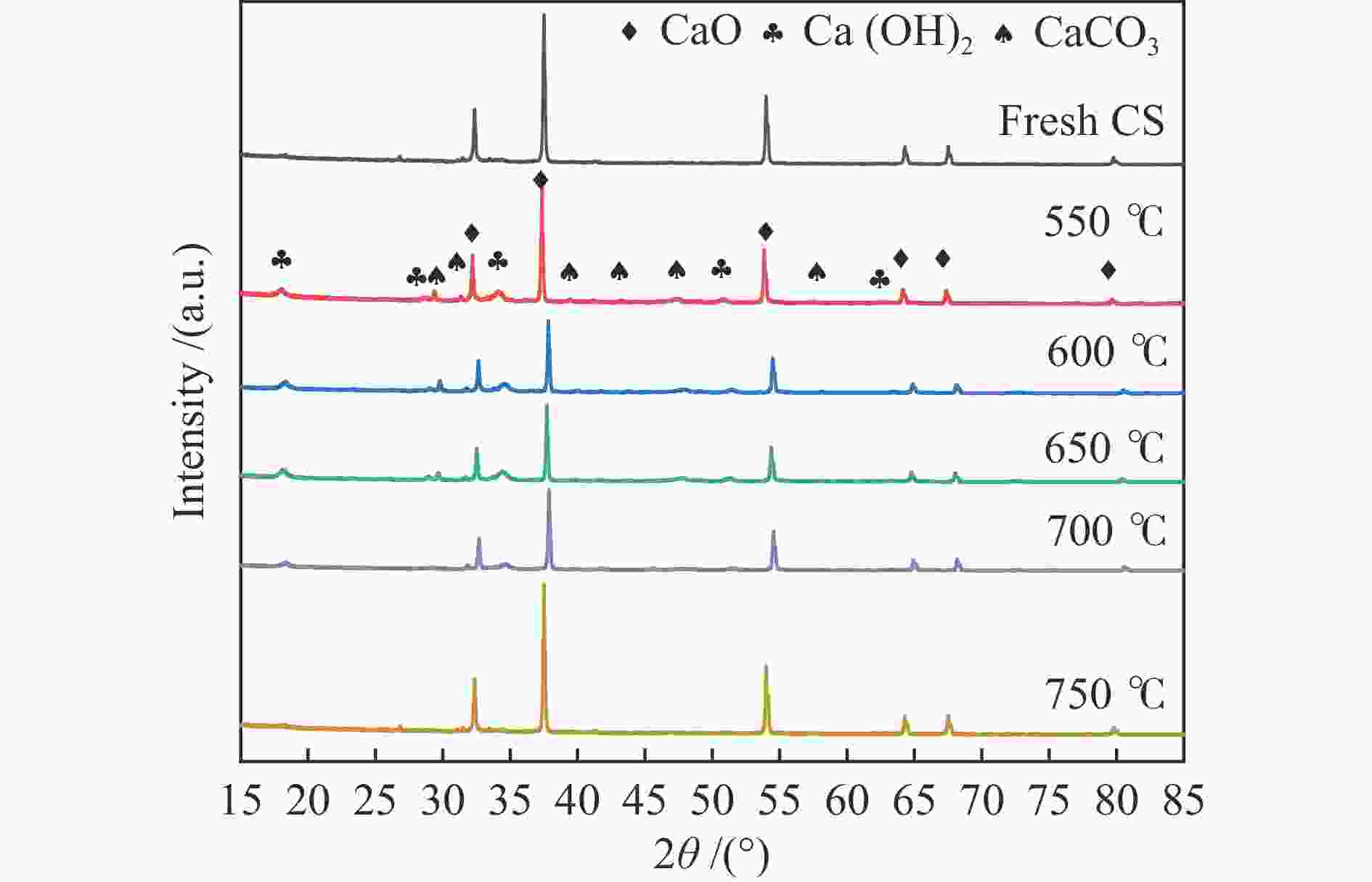
Sample Mass fraction w/% CaO MgO SiO2 Al2O3 Fe2O3 TiO2 Na2O others Carbide slag 90.55 0.12 2.81 3.97 0.45 0.49 0.03 1.58 表 3 吸附剂循环反应后的气体产物特性
Table 3 Characteristics of gas products after cyclic reaction of adsorbent
Cycles 1 2 3 4 5 Gas production rate
w/%39.38 39.20 40.36 38.92 37.23 Hydrogen production /
(mL·g−1 biomass)154.34 192.64 188.16 161.28 159.49 -
[1] UDOMSIRICHAKORN J, BASU P, SALAM P A, ACHARYA B. Effect of CaO on tar reforming to hydrogen-enriched gas with in-process CO2 capture in a bubbling fluidized bed biomass steam gasifier[J]. Int J Hydrog Energy,2013,38(34):14495−14504. doi: 10.1016/j.ijhydene.2013.09.055 [2] DUAN W, YU Q. Thermodynamic analysis of hydrogen-enriched syngas generation coupled with in situ CO2 capture using chemical looping gasification method[J]. J Therm Anal Calorim,2017,131(2):1671−1680. [3] DONG J, NZIHOU A, CHI Y, WEISS-HORTALA E, NI M, LYCZKO N, TANG Y, DUCOUSSO M. Hydrogen-rich gas production from steam gasification of bio-char in the presence of CaO[J]. Waste Biomass Valori,2016,8(8):2735−2746. [4] LI B, MAGOUA MBEUGANG C F, HUANG Y, LIU D, WANG Q, ZHANG S. A review of CaO based catalysts for tar removal during biomass gasification[J]. Energy,2022,244:1. [5] 耿一琪, 郭彦霞, 樊飙, 程芳琴, 成怀刚. CaO基吸附剂捕集CO2及其抗烧结改性研究进展[J]. 燃料化学学报,2021,49(7):998−1013. doi: 10.1016/S1872-5813(21)60040-3GENG Yi-qi, GUO Yan-xia, FAN Biao, CHENG Fang-qin, CHENG Huai-gang. Research progress of calcium-based adsorbents for CO2 capture and anti-sintering modification[J]. J Fuel Chem Technol,2021,49(7):998−1013. doi: 10.1016/S1872-5813(21)60040-3 [6] ZHANG H, JIANG T, YASEEN H A S M, ZHAO Y, WANG S, MA X. Pelletization and attrition of CaO‐based adsorbent for CO2 capture[J]. Asia-Pac J Chem Eng,2021,16(4):2656-1−2656-19. [7] XU Y, LUO C, ZHENG Y, DING H, WANG Q, SHEN Q, LI X, ZHANG L. Characteristics and performance of CaO-based high temperature CO2 sorbents derived from a sol-gel process with different supports[J]. RSC Adv,2016,6(83):79285−79296. doi: 10.1039/C6RA15785H [8] WANG S, SHEN H, FAN S, ZHAO Y, MA X, GONG J. Enhanced CO2 adsorption capacity and stability using CaO-based adsorbents treated by hydration[J]. AIChE J,2013,59(10):3586−3593. doi: 10.1002/aic.14126 [9] YANG H, WANG D, LI B, ZENG Z, QU L, ZHANG W, CHEN H. Effects of potassium salts loading on calcium oxide on the hydrogen production from pyrolysis-gasification of biomass[J]. Bioresour Technol,2018,249:744−750. doi: 10.1016/j.biortech.2017.10.083 [10] XU A, ZHOU W, ZHANG X, ZHAO B, CHEN L, SUN L, DING W, YANG S, GUAN H, BAI B. Gas production by catalytic pyrolysis of herb residues using Ni/CaO catalysts[J]. J Anal Appl Pyrolysis,2018,130:216−223. doi: 10.1016/j.jaap.2018.01.006 [11] LIU F, LI W, LIU B, LI R. Synthesis, characterization, and high temperature CO2 capture of new CaO based hollow sphere sorbents[J]. J Mater Chem A,2013,1(27):8037. doi: 10.1039/c3ta11369h [12] 刘小通. 改性钙基CO2高温吸附剂的研究[D]. 西安: 西北大学, 2017.LIU Xiao-tong . Study of modified CaO-based sorbents for CO2 at high temperture[D]. Xi 'an : Northwest University, 2017. [13] LV S Z, ZHAO S Y, LIU M M, WU P P. Preparation of calcium carbonate by calcium carbide residue[J]. Adv Mat Res,2013,864−867:1963−1967. [14] LI Y, WANG W, CHENG X, SU M, MA X, XIE X. Simultaneous CO2/HCl removal using carbide slag in repetitive adsorption/desorption cycles[J]. Fuel,2015,142:21−27. doi: 10.1016/j.fuel.2014.10.071 -




 下载:
下载: